New transitory form of silica observed
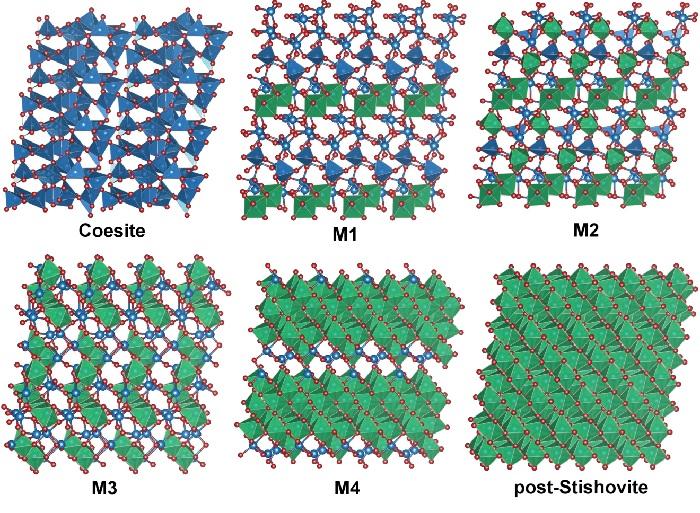
A simulated visual representation of the structural transition from coesite to post-stishovite. The silicon atoms (blue spheres) surrounded by four oxygen atoms (red spheres) are shown as blue tetrahedrons. The silicon atoms surrounded by six oxygen atoms are shown as green octahedrons. The intermediate phases are not filled in with color, showing the four stages that are neither all-blue like coesite nor all-green like post-stishovite. This image is provided courtesy of Ho-Kwang Mao. Credit: Ho-Kwang Mao
Silicon dioxide, commonly called silica, is one of the most-abundant natural compounds and a major component of the Earth's crust and mantle. It is well-known even to non-scientists in its quartz crystalline form, which is a major component of sand in many places. It is used in the manufacture of microchips, cement, glass, and even some toothpaste.
Silica's various high-pressure forms make it an often-used study subject for scientists interested in the transition between different chemical phases under extreme conditions, such as those mimicking the deep Earth.
The first-discovered high-pressure, high-temperature denser form, or phase, of silica is called coesite, which, like quartz, consists of building blocks of silicon atoms surrounded by four oxygen atoms. Under greater pressures and temperatures, it transforms into an even denser form called stishovite, with silicon atoms surrounded by six oxygen atoms.
The transition between these phases was crucial for learning about the pressure gradient of the deep Earth and the four-to-six configuration shift has been of great interest to geoscientists. Experiments have revealed even higher-pressure phases of silica beyond these two, sometimes called post-stishovite.
A chemical phase is a distinctive and uniform configuration of the molecules that make up a substance. Changes in external conditions, such as temperature and pressure, can induce a transition from one phase to another, not unlike water freezing into ice or boiling into steam.
The team, including Carnegie's Qingyang Hu, Jinfu Shu, Yue Meng, Wenge Yang, and Ho-Kwang, “Dave” Mao, demonstrated that under a range from 257,000 to 523,000 times normal atmospheric pressure (26 to 53 gigapascals), a single crystal of coesite transforms into four new, co-existing crystalline phases before finally recombining into a single phase that is denser than stishovite, sometimes called post-stishovite, which is the team's fifth newly discovered phase. This transition takes place at room temperature, rather than the extreme temperatures found deep in the earth.
Scientists previously thought that this intermediate was amorphous, meaning that it lacked the long-range order of a crystalline structure. This new study uses superior x-ray analytical probes to show otherwise–they are four, distinct, well-crystalized phases of silica without amorphization. Advanced theoretical calculations performed by the team provided detailed explanations of the transition paths from coesite to the four crystalline phases to post-stishovite.
“Scientists have long debated whether a phase exists between the four- and six-oxygen phases,” Mao said. “These newly discovered four transition phases and the new phase of post-stishovite we discovered show the missing link for which we've been searching.”
###
The paper's other co-authors are Adam Cadien of George Mason University and Howard Sheng of both the Center for High Pressure Science and Technology Advanced Research in Shanghai, China, and George Mason University.
This work was supported by the NSF. HPCAT operations are supported by CIW, CDAC, UNLV and LLNL through funding from DOE-NNSA and DOE-BES, with partial instrumentation funding by NSF.
The Carnegie Institution for Science (carnegiescience.edu) is a private, nonprofit organization headquartered in Washington, D.C., with six research departments throughout the U.S. Since its founding in 1902, the Carnegie Institution has been a pioneering force in basic scientific research. Carnegie scientists are leaders in plant biology, developmental biology, astronomy, materials science, global ecology, and Earth and planetary science.
Media Contact
All latest news from the category: Materials Sciences
Materials management deals with the research, development, manufacturing and processing of raw and industrial materials. Key aspects here are biological and medical issues, which play an increasingly important role in this field.
innovations-report offers in-depth articles related to the development and application of materials and the structure and properties of new materials.
Newest articles

Bringing bio-inspired robots to life
Nebraska researcher Eric Markvicka gets NSF CAREER Award to pursue manufacture of novel materials for soft robotics and stretchable electronics. Engineers are increasingly eager to develop robots that mimic the…

Bella moths use poison to attract mates
Scientists are closer to finding out how. Pyrrolizidine alkaloids are as bitter and toxic as they are hard to pronounce. They’re produced by several different types of plants and are…

AI tool creates ‘synthetic’ images of cells
…for enhanced microscopy analysis. Observing individual cells through microscopes can reveal a range of important cell biological phenomena that frequently play a role in human diseases, but the process of…





















