
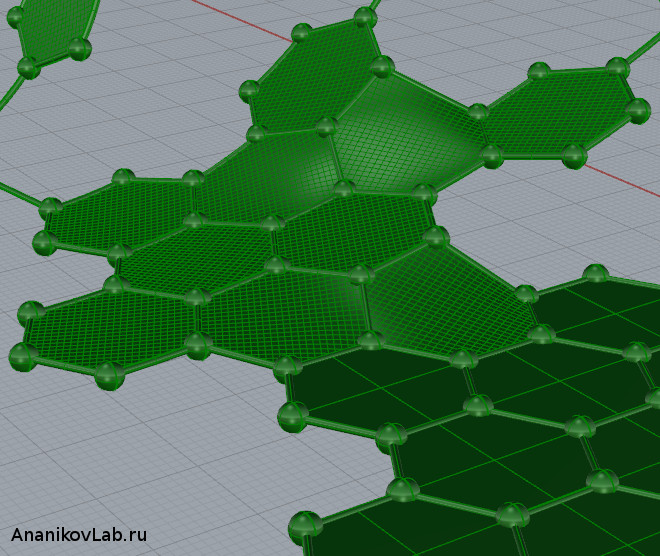
Folding and transforming carbon rings.
Copyright : Ananikov Laboratory (AnanikovLab.ru)
Graphene “cut and paste” with metal nanoparticles was carried out under microwave irradiation. The study revealed unique processes occurring on the carbon layers under the influence of metal nanoparticles heated by microwave irradiation. Understanding the processes taking place in Metal/Carbon systems is crucial for development of new generation of highly efficient catalysts for organic synthesis and chemical industry. The authors described the key transformations responsible for catalyst evolution in connection with preparation of nanostructured Metal/Carbon systems [1].
The study, carried out in the laboratory of Prof. V.P.Ananikov at Zelinsky Institute of Organic Chemistry of Russian Academy of Sciences, discovered a variety of processes occurring on the surface of carbon material upon a contact with hot metal nanoparticles. Metal nanoparticles, heated by microwave radiation, caused significant morphological changes of the carbon surface: formation of patterns of pits and channels, penetration inside the carbon material and direct growth of carbon nanotubes.
As it is widely accepted now, discovery and systematic study of carbon nanotubes was one of the prominent groundbreaking points at the beginning of nanotechnology era. Carbon nanotubes are nanoscale tubular structures consisting of carbon atoms arranged in the interconnected six-membered rings within a cylindrical wall.
From a certain point of view carbon nanotube may be represented as a graphene sheet (flat sheet of monoatomic thickness) rolled into a cylinder and carbon-glued at the edges. Direct access to carbon nanotubes starting from graphene (and especially starting from much cheaper precursor – graphene layers in graphite) would be an outstanding process of great practical interest. The questions appear: how easy is to cut graphite sheets and to roll them up? Does it violate thermodynamic factors?
The present study, published in the ACS Catalysis journal, discovered a series of processes mediated in metal-carbon systems under microwave irradiation. “Cutting” of carbon slices by hot metal particles was clearly observed by field-emission scanning electron microscopy (FE-SEM). “Pasting” of carbon atoms into a new location has resulted in a growth of carbon nanotubes on the surface of graphite – the process was also observed in the experiment under an inert atmosphere.
In the theoretical modeling the authors considered the following possibility: at the first stages graphene sheet can be cut into nanoribbons of one aromatic ring wide (Figure 1). Then, each nanoribbon is rolled into cycloparaphenylenes – these molecules are known and were described previously. On the later stages, cycloparaphenylene rings are joined together to form the nanotube. Important stages of this process were modeled by quantum chemical calculations involving density functional theory.
As shown by theoretical modeling, the energy of such process depends strongly on the initial state of the edges of graphene sheet. If the edges are capped with hydrogen (reaction 1, Figure 2), the overall process of nanotube formation reaction is accompanied by releasing of 20 hydrogen molecules and it is energetically unfavorable (increase in energy is ~2.5 kcal/mol per one carbon atom).
Reaction (2) involves partially hydrogenated graphene edges and it is energetically more favorable (energy decrease is ~1.5 kcal/mol per one carbon atom). The most favorable process from thermodynamic point of view is the formation of a nanotube from a fully dehydrogenated graphene sheet (reaction 3). This process was accompanied by an energy decrease of ~4.6 kcal/mol per one carbon atom.
Important findings, described in the article, deal with transformation of carbon support in Metal/Carbon catalysts. For a long time it was considered that carbon support is an inert (innocent) material used only for supporting (anchoring) of metal nanoparticles. The present study has clearly shown that it is not always the case. Metal particles do interact with carbon support and the interaction leads to amazing modification of the morphology of Metal/Carbon systems. Understanding the nature of this interaction plays a key role in developing efficient and stable catalytic systems. Evolution of the catalyst during chemical transformation may be responsible for deactivation of the catalyst and loss of catalytic activity.
Reference
[1] Evgeniy O. Pentsak, Evgeniy G. Gordeev, Valentine P. Ananikov, «Noninnocent Nature of Carbon Support in Metal/Carbon Catalysts: Etching/Pitting vs Nanotube Growth under Microwave Irradiation», ACS Catalysis, 2014, Vol. 4, pp. 3806−3814.
DOI: 10.1021/cs500934g.
On-line link: http://dx.doi.org/10.1021/cs500934g
Cover picture: http://pubs.acs.org/action/showLargeCover?jcode=accacs&vol=4&issue=11
Associated links
http://AnanikovLab.ru












