New method converts methane in natural gas to methanol at room temperature
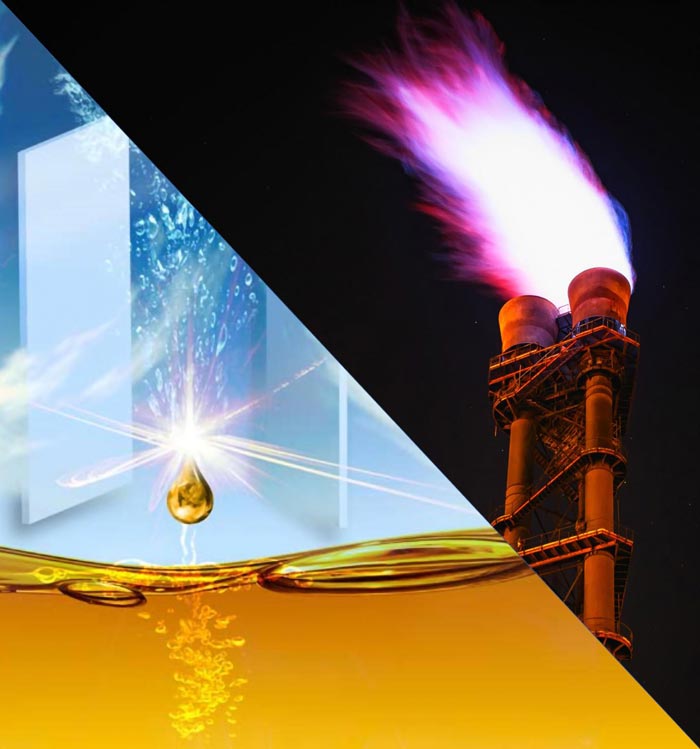
Burning methane in natural gas contributes to carbon emissions, but methane converted to liquid methanol is a cleaner fuel.
Credit: Aditya Prajapati and Meenesh Singh/UIC
Fueling the ‘methanol economy’ of the future
Researchers at the University of Illinois Chicago have discovered a way to convert the methane in natural gas into liquid methanol at room temperature.
This discovery, reported in the journal Proceedings of the National Academy of Sciences, could potentially provide a cleaner energy source for many of our everyday activities.
When burned, natural gas — the fuel used to heat homes, cook food and generate electricity — produces carbon dioxide, a powerful greenhouse gas.
According to the U.S. Energy Information Administration, the U.S. consumed approximately 31 trillion cubic feet of natural gas in 2019, contributing roughly 1.6 gigatons of carbon dioxide to the atmosphere.
A better way to use natural gas would be to convert it to methanol, a liquid fuel that burns more cleanly and can be used to produce gasoline and plastics. But converting the methane found in natural gas into methanol requires a lot of heat and pressure and generates a significant amount of carbon dioxide itself.
“Researchers have been interested in ways to convert methane to methanol at ambient temperatures to sidestep all the heat and pressure that is currently required in industrial processes to perform this conversion,” said Meenesh Singh, assistant professor of chemical engineering at the UIC College of Engineering and corresponding author of the paper.
Methanol also is thought to be the “fuel in the future,” driving a “methanol economy” where it replaces fossil fuels in transportation, energy storage and as the dominant precursor material for synthetic chemicals and other products. Methanol is currently used in fuel cell technology that powers some city buses and other vehicles. Its lower emission potentials and higher volumetric energy density make it an attractive alternative to fossil fuels, Singh said.
“Besides being a cleaner-burning fuel, methane can also be stored safely in regular containers, unlike natural gas, which has to be stored under pressure and which is much more expensive,” Singh said.
High amounts of heat and pressure are required to break the hydrocarbon bonds in methane gas, the first step in producing methanol. But Singh and UIC graduate student Aditya Prajapati have identified a catalyst material that helps bring down the energy needed to break these bonds so that the reaction can take place at room temperature.
“We have been able to reduce the temperature of the industrial process from more than 200 degrees Celsius to room temperature, which is around 20 degrees Celsius,” Prajapati said.
Their catalyst is composed of titanium and copper. The catalyst, together with a small amount of electricity, facilitates the breaking of the hydrocarbon bonds of methane and the formation of methanol. The process uses much less energy than traditional methods, and because it doesn’t require machinery to produce high pressure and heat, it can be set up quickly and inexpensively.
“Our process doesn’t need to be centralized,” Singh said. “It can be implemented in a space as small as a van and is portable for distributed utilization of natural gas and manufacturing of methanol.”
Singh and colleagues have filed a provisional patent for the process and expect that it could convert a few liters of methanol a day. The patent is being managed through the UIC Office of Technology Management.
###
Brianna Collins and Jason Goodpaster of the University of Minnesota are co-authors on the paper.
Media Contact
All latest news from the category: Power and Electrical Engineering
This topic covers issues related to energy generation, conversion, transportation and consumption and how the industry is addressing the challenge of energy efficiency in general.
innovations-report provides in-depth and informative reports and articles on subjects ranging from wind energy, fuel cell technology, solar energy, geothermal energy, petroleum, gas, nuclear engineering, alternative energy and energy efficiency to fusion, hydrogen and superconductor technologies.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…





















