Texas A&M engineers grow 3D bioprinted blood vessel
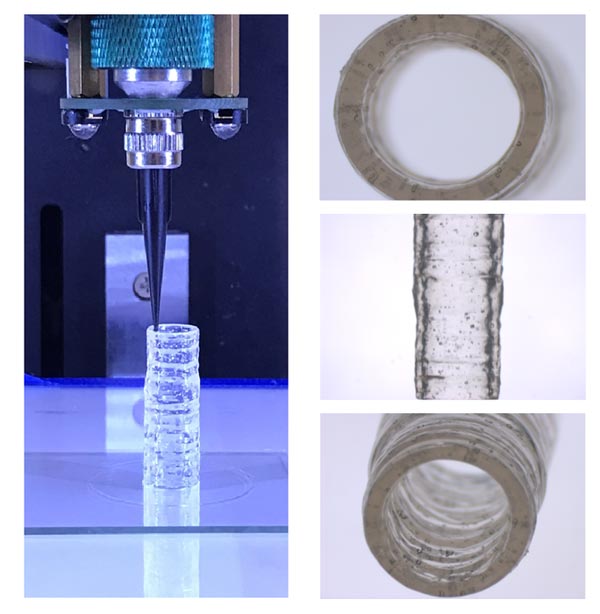
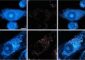
These images depict the method used to fabricate the 3D bioprinted vascular model with native endothelial and vascular smooth muscle cells.
Credit: Dr. Akhilesh Gaharwar/Texas A&M Engineering
Texas A&M researchers designed a blood vessel model that mimics its state of health and disease, paving the way for cardiovascular drug advancements with better precision.
A team in the Department of Biomedical Engineering, co-led by associate professor Dr. Akhilesh Gaharwar and assistant professor Dr. Abhishek Jain, has designed a 3D-bioprinted model of a blood vessel that mimics its state of health and disease, thus paving the way for possible cardiovascular drug advancements with better precision.
Vascular diseases such as aneurysms, peripheral artery disease and clots inside blood vessels account for 31% of global deaths. Despite this clinical burden, cardiovascular drug advancements have slowed over the past 20 years. The decrease in cardiovascular therapeutic development is attributed to the lack of efficiency in converting possible treatments into approved methods, specifically due to the discrepancy between studies that take place outside the body compared to inside.
The team’s research at Texas A&M University aims to remodel current methodologies to minimize this gap and improve the translatability of these techniques by directing 3D bioprinting toward vascular medicine. Gaharwar is a biomaterials expert and has developed novel bioinks that offer unprecedented biocompatibility and control of mechanical properties needed to print blood vessels, whereas Jain’s expertise lies in creating biomimetic in vitro models of vascular and hematological diseases. This interdisciplinary and collaborative project was recently published in the journal Advanced Healthcare Materials.
Bioprinting in 3D is an advanced manufacturing technique capable of producing unique, tissue-shaped constructs in a layer-by-layer fashion with embedded cells, making the arrangement more likely to mirror the native, multicellular makeup of vascular structures. A range of hydrogel bioinks was introduced to design these structures; however, there is a limitation in available bioinks that can mimic the vascular composition of native tissues. Current bioinks lack high printability and are unable to deposit a high density of living cells into complex 3D architectures, making them less effective.
To overcome these shortcomings, Gaharwar and Jain developed a new nanoengineered bioink to print 3D, anatomically accurate, multicellular blood vessels. Their approach offers improved real-time resolution for both macro-structure and tissue-level micro-structure, something that currently is not possible with available bioinks.
“A remarkably unique characteristic of this nanoengineered bioink is that regardless of cell density, it demonstrates a high printability and ability to protect encapsulated cells against high shear forces in the bioprinting process,” Gaharwar said. “Remarkably, 3D-bioprinted cells maintain a healthy phenotype and remain viable for nearly one month postfabrication.”
Leveraging these unique properties, the nanoengineered bioink is printed into 3D cylindrical blood vessels, consisting of living co-cultures of endothelial cells and vascular smooth muscle cells, which provides researchers the opportunity to model vascular function and disease impact.
This 3D-bioprinted vessel provides a potential tool to understand vascular disease pathophysiology and assess therapeutics, toxins or other chemicals in preclinical trials.
Other project collaborators include Dr. John Cooke from the Houston Methodist Research Institute and Dr. Javier Jo from the University of Oklahoma. This research is funded through grants from the National Institutes of Health, the National Science Foundation and the Texas A&M President’s Excellence Fund.
A team in the Department of Biomedical Engineering, co-led by associate professor Dr. Akhilesh Gaharwar and assistant professor Dr. Abhishek Jain, has designed a 3D-bioprinted model of a blood vessel that mimics its state of health and disease, thus paving the way for possible cardiovascular drug advancements with better precision.
Vascular diseases such as aneurysms, peripheral artery disease and clots inside blood vessels account for 31% of global deaths. Despite this clinical burden, cardiovascular drug advancements have slowed over the past 20 years. The decrease in cardiovascular therapeutic development is attributed to the lack of efficiency in converting possible treatments into approved methods, specifically due to the discrepancy between studies that take place outside the body compared to inside.
The team’s research at Texas A&M University aims to remodel current methodologies to minimize this gap and improve the translatability of these techniques by directing 3D bioprinting toward vascular medicine. Gaharwar is a biomaterials expert and has developed novel bioinks that offer unprecedented biocompatibility and control of mechanical properties needed to print blood vessels, whereas Jain’s expertise lies in creating biomimetic in vitro models of vascular and hematological diseases. This interdisciplinary and collaborative project was recently published in the journal Advanced Healthcare Materials.
Bioprinting in 3D is an advanced manufacturing technique capable of producing unique, tissue-shaped constructs in a layer-by-layer fashion with embedded cells, making the arrangement more likely to mirror the native, multicellular makeup of vascular structures. A range of hydrogel bioinks was introduced to design these structures; however, there is a limitation in available bioinks that can mimic the vascular composition of native tissues. Current bioinks lack high printability and are unable to deposit a high density of living cells into complex 3D architectures, making them less effective.
To overcome these shortcomings, Gaharwar and Jain developed a new nanoengineered bioink to print 3D, anatomically accurate, multicellular blood vessels. Their approach offers improved real-time resolution for both macro-structure and tissue-level micro-structure, something that currently is not possible with available bioinks.
“A remarkably unique characteristic of this nanoengineered bioink is that regardless of cell density, it demonstrates a high printability and ability to protect encapsulated cells against high shear forces in the bioprinting process,” Gaharwar said. “Remarkably, 3D-bioprinted cells maintain a healthy phenotype and remain viable for nearly one month postfabrication.”
Leveraging these unique properties, the nanoengineered bioink is printed into 3D cylindrical blood vessels, consisting of living co-cultures of endothelial cells and vascular smooth muscle cells, which provides researchers the opportunity to model vascular function and disease impact.
This 3D-bioprinted vessel provides a potential tool to understand vascular disease pathophysiology and assess therapeutics, toxins or other chemicals in preclinical trials.
Other project collaborators include Dr. John Cooke from the Houston Methodist Research Institute and Dr. Javier Jo from the University of Oklahoma. This research is funded through grants from the National Institutes of Health, the National Science Foundation and the Texas A&M President’s Excellence Fund.
DOI: https://doi.org/10.1002/adhm.202101141
Method of Research: News article
Subject of Research: Not applicable
Article Title: 3D Bioprinted Multicellular Vascular Models
Article Publication Date: 26-Jul-2021
All latest news from the category: Medical Engineering
The development of medical equipment, products and technical procedures is characterized by high research and development costs in a variety of fields related to the study of human medicine.
innovations-report provides informative and stimulating reports and articles on topics ranging from imaging processes, cell and tissue techniques, optical techniques, implants, orthopedic aids, clinical and medical office equipment, dialysis systems and x-ray/radiation monitoring devices to endoscopy, ultrasound, surgical techniques, and dental materials.
Newest articles

High-energy-density aqueous battery based on halogen multi-electron transfer
Traditional non-aqueous lithium-ion batteries have a high energy density, but their safety is compromised due to the flammable organic electrolytes they utilize. Aqueous batteries use water as the solvent for…

First-ever combined heart pump and pig kidney transplant
…gives new hope to patient with terminal illness. Surgeons at NYU Langone Health performed the first-ever combined mechanical heart pump and gene-edited pig kidney transplant surgery in a 54-year-old woman…

Biophysics: Testing how well biomarkers work
LMU researchers have developed a method to determine how reliably target proteins can be labeled using super-resolution fluorescence microscopy. Modern microscopy techniques make it possible to examine the inner workings…