Why remdesivir does not fully stop the coronavirus
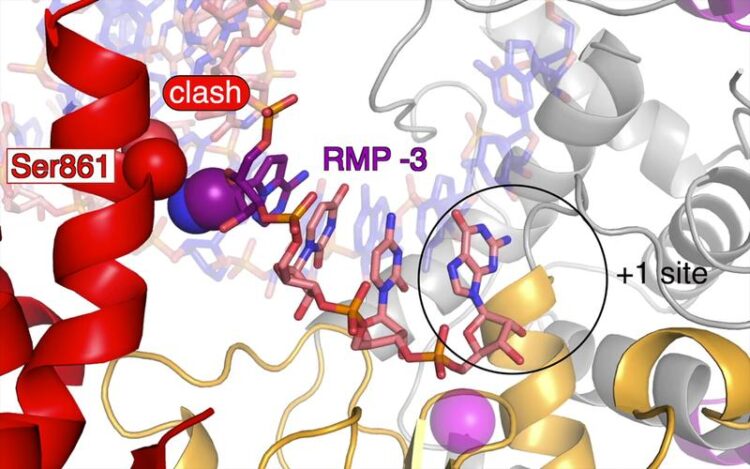
The Covid-19 drug Remdesivir (purple) is incorporated into the new RNA chain during the copying process and suppresses the duplication of the coronavirus genome.
Hauke Hillen, Goran Kokic and Patrick Cramer / Max Planck Institute for Biophysical Chemistry
Remdesivir is the first drug against Covid-19 to be conditionally approved in Europe and the United States. The drug is designed to suppress the rapid replication of the SARS-CoV-2 virus in human cells by blocking the viral copying machine, called RNA polymerase. Researchers at the Max Planck Institute (MPI) for Biophysical Chemistry in Göttingen and the University of Würzburg have now elucidated how remdesivir interferes with the viral polymerase during copying and why it does not inhibit it completely. Their results explain why the drug has a rather weak effect.
“After complicated studies, we come to a simple conclusion,” Max Planck Director Patrick Cramer says. “Remdesivir does interfere with the polymerase while doing its work, but only after some delay. And the drug does not fully stop the enzyme.”
At the pandemic’s beginning, Cramer’s team at the MPI for Biophysical Chemistry had elucidated how the coronavirus duplicates its RNA genome. For the pathogen this is a colossal task as its genome comprises around 30,000 RNA building blocks, making it particularly long. To elucidate remdesivir’s mechanism of action, Cramer’s team collaborated with Claudia Höbartner’s group. The latter produced special RNA molecules for the structural and functional studies. “Remdesivir’s structure resembles that of RNA building blocks,” explains Höbartner, a professor of chemistry at the University of Würzburg. The polymerase is thereby misled and integrates the substance into the growing RNA chain.
Pausing instead of blocking
After remdesivir had been incorporated into the viral genome, the researchers examined the polymerase-RNA complexes using biochemical methods and cryo-electron microscopy. They discovered that the copying process pauses precisely when three more building blocks have been added after remdesivir was incorporated into the RNA chain. “The polymerase does not allow the installation of a fourth one. This pausing is caused by only two atoms in the structure of remdesivir that get hooked at a specific site on the polymerase. However, remdesivir does not fully block RNA production. Often, the polymerase continues its work after correcting the error,” explains Goran Kokic, a research associate in Cramer’s lab, who together with Hauke Hillen, Dimitry Tegunov, Christian Dienemann, and Florian Seitz, had conducted the crucial experiments. They all are first authors of the publication about this work recently published in the scientific magazine Nature Communications.
Understanding how remdesivir works opens up new opportunities for scientists to tackle the virus. “Now that we know how remdesivir inhibits the corona polymerase, we can work on improving the substance and its effect. In addition, we want to search for new compounds that stop the viral copying machine,” Max Planck Director Cramer says. “The vaccinations now underway are essential to bring the pandemic under control. But we also need to develop effective drugs that mitigate Covid-19 disease progression in the event of infection.”
Wissenschaftliche Ansprechpartner:
Prof. Dr. Patrick Cramer
Department of Molecular Biology, Max Planck Institute for Biophysical Chemistry
phone: +49 551 201-2800
e-mail: patrick.cramer@mpibpc.mpg.de
Prof. Dr. Claudia Höbartner
Institute for Organic Chemistry, University of Würzburg
phone: +49 931 31-89693
e-mail: claudia.hoebartner@uni-wuerzburg.de
Originalpublikation:
Goran Kokic, Hauke Sven Hillen, Dimitry Tegunov, Christian Dienemann, Florian Seitz, Jana Schmitzova, Lucas Farnung, Aaron Siewert, Claudia Hoebartner, Patrick Cramer: Mechanism of SARS-CoV-2 polymerase inhibition by remdesivir. Nature Communications 12, 279 (2021), doi: 10.1038/s41467-020-20542-0
Weitere Informationen:
https://www.mpibpc.mpg.de/17619258/pr_2101 − Original press release
https://www.mpibpc.mpg.de/cramer − Website of Patrick Cramer’s Department of Molecular Biology, Max Planck Institute for Biophysical Chemistry
https://go.uniwue.de/hoebartner-group − Website of Claudia Höbartner’s group Organic and biomolecular chemistry, University of Würzburg
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Bringing bio-inspired robots to life
Nebraska researcher Eric Markvicka gets NSF CAREER Award to pursue manufacture of novel materials for soft robotics and stretchable electronics. Engineers are increasingly eager to develop robots that mimic the…

Bella moths use poison to attract mates
Scientists are closer to finding out how. Pyrrolizidine alkaloids are as bitter and toxic as they are hard to pronounce. They’re produced by several different types of plants and are…

AI tool creates ‘synthetic’ images of cells
…for enhanced microscopy analysis. Observing individual cells through microscopes can reveal a range of important cell biological phenomena that frequently play a role in human diseases, but the process of…





















