The working of a molecular string phone
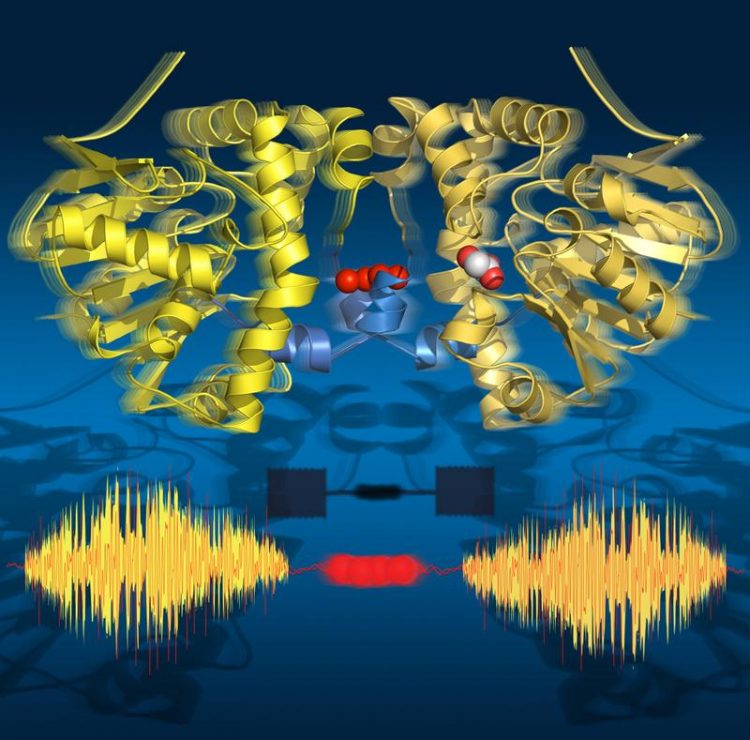
Time-lapse images show that the enzyme ‘breathes’ during turnover: it expands and contracts aligned with the catalytic sub-steps. Its two halves communicate via a string of water molecules. Jörg Harms / MPSD
This time-lapse sequence of structures reveals dynamic motions as a fundamental element in the molecular foundations of biology.
All life is dynamic and so are its molecular building blocks. The motions and structural changes of biomolecules are fundamental to their functions. However, understanding these dynamic motions at a molecular level is a formidable challenge. How is a protein able to accelerate a chemical reaction, which would take years to proceed without help?
To this end the researchers turned to an enzyme that splits the strongest single-bond in organic chemistry: the C-F bond. Fluorinated carbons can be found in materials such as Teflon or GoreTex and in many pharmaceuticals and pesticides.
Fluorinated compounds have a particular influence in climate change, exceeding the effectiveness of CO2 by orders magnitude. Therefore, the ability to better understand and eventually control the turnover of C-F bonds is of particular interest to climate change and bioremediation.
The researchers used time-resolved X-ray crystallography to take molecular snapshots during the turnover reaction of this natural enzyme at physiological temperatures.
This time-lapse movie revealed eighteen time points from 30 milliseconds to 30 seconds, covering all key catalytic states that lead to the breaking of the C-F bond. Surprisingly, the movie also shows that the enzyme ‘breathes’ during turnover, that is it expands and contracts aligned with the catalytic sub-steps.
Strikingly, the two halves of the enzyme communicate with each other via a string of water molecules that connects both halves. This water network allows the two halves to 'talk' to one another and share information about their catalytic state. This is crucial to the enzyme’s function as only one half of the enzyme can ever be active at a given time.
These dynamic changes have proven crucial to the enzyme’s function. The researchers expect many other systems to exploit similar mechanisms for their activities.
Prof. Dr. R.J. D. Miller (Corresponding Author) Email: dwayne.miller@mpsd.mpg.de
Prof. Dr. Emil Pai Email: emil.pai@utoronto.ca
Science article: https://science.sciencemag.org/content/365/6458/1167
http://www.mpsd.mpg.de/333685/2019-09-mehrabi-string-phone?c=17189
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Bringing bio-inspired robots to life
Nebraska researcher Eric Markvicka gets NSF CAREER Award to pursue manufacture of novel materials for soft robotics and stretchable electronics. Engineers are increasingly eager to develop robots that mimic the…

Bella moths use poison to attract mates
Scientists are closer to finding out how. Pyrrolizidine alkaloids are as bitter and toxic as they are hard to pronounce. They’re produced by several different types of plants and are…

AI tool creates ‘synthetic’ images of cells
…for enhanced microscopy analysis. Observing individual cells through microscopes can reveal a range of important cell biological phenomena that frequently play a role in human diseases, but the process of…





















