Smarter catalysts through ‘induced activation’
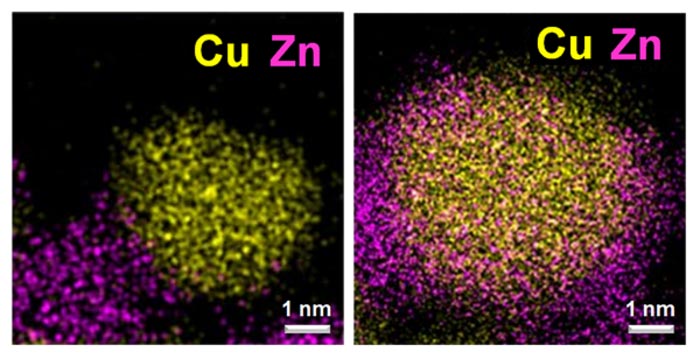
Scanning transmission electron microscopy images of catalysts metallic copper (yellow) and zinc oxide (pink/orange). In the image on the left, metallic Cu and Zn oxide are mostly present as separate particles after activation with H2. The image on the right shows Zn oxide decorating metallic Cu particles after “induced activation” with H2/CH3OH/H2O.
Courtesy of Xuan Tang and Prof. Sheng Dai, East China University of Science and Technology
New Lehigh University–East China University of Science & Technology research collaboration proposes novel method of molecular-level control to double the efficiency of widely used industrial catalysts.
The science of catalysis—the acceleration of a chemical reaction—is perhaps not the most recognizable branch of study, but it is absolutely embedded into the fabric of modern society.
The development and production of fuels, chemicals, pharmaceuticals and other goods depend on catalysis. Catalysis plays a critical role in energy generation and the mitigation of humanity’s impact on the environment, and is involved in the manufacturing of some 25 percent of all industrial products in the U.S. From a consumer’s perspective, if a thing is made, worn, lived in, played with, driven upon, or otherwise used by people, catalysis likely plays a fundamental role in its origin story.
Research in the field of catalysis enables new and improved products and more efficient ways of doing and manufacturing, well, just about everything. But with such deep entanglement in the world around us, advancement in industrial catalysis can be costly in a macroeconomic sense—wholesale changes that require a “rip and replace” strategy do not sit well with firms and supply chains that power and provision our modern economy.
In a paper published online today (20 January 2022) in Nature Catalysis, researchers from Lehigh University, in collaboration with colleagues from the East China University of Science and Technology (ECUST), propose a novel method of significantly enhancing the catalytic efficiency of materials already in broad commercial usage, a process they have termed “induced activation.”
The article, “Induced activation of the commercial Cu/ZnO/Al2O3 catalyst for the steam reforming of methanol,” The research team, supported by the National Natural Science Foundation of China and the U.S. Department of Energy’s Office of Science, includes Israel E. Wachs, the G. Whitney Snyder Professor of Chemical and Biomolecular Engineering at Lehigh University, PhD student Tiancheng Pu of Lehigh’s Operando Molecular Spectroscopy and Catalysis Research Lab, and Minghui Zhu, a 2016 Lehigh PhD who now serves as a professor of chemical engineering at ECUST. Other collaborating ECUST researchers include Didi Li, Fang Xu, Xuan Tang, Sheng Dai, Xianglin Liu, Pengfei Tian, Fuzhen Xuan, and Zhi Xu.
Induced activation: a game changer in the control of catalytic surface
“The surface structure of heterogeneous catalysts is closely associated with their catalytic performance,” explains Wachs. “Current efforts for structural modification mainly focus on improving catalyst synthesis. Induced activation, on the other hand, takes a different approach—manipulating the catalyst surface by controlling the composition of reducing agents at the catalyst activation stage where the catalyst is transformed to its optimum state.”
The team says that the use of the “tried and true” industrial catalytic material copper/zinc oxide/aluminum oxide (Cu/ZnO/AlO3) enables firms to take advantage of the breakthrough without the need for a costly retooling.
“This development effectively doubles the catalytic efficiency of these materials, enhancing their productivity and extending the life of the catalyst,” Wachs continues. “And importantly, induced activation can provide significant benefit to industry without shutting down a chemical plant—or the building of a new and costly one.”
For more, review the research as published in Nature Catalysis. (https://doi.org/10.1038/s41929-021-00729-4)
About Israel Wachs
In a career spanning three decades, Wachs has earned international renown for research into fundamental and applied aspects of heterogeneous catalysis.
His research focuses on the catalysis science of mixed metal oxides (supported metal oxides, bulk metal oxides, polyoxometalates, zeolites and molecular sieves) for numerous catalytic applications (selective oxidation for manufacture of value-added chemicals, environmental catalysis (selective catalytic reduction of NOx with ammonia), hydrocarbon conversion by solid acid catalysts for increased fuel energy content, oxidative coupling of methane to ethylene and ethane, ethylene oxidation to ethylene oxide, dimerization of ethylene to butene, olefin polymerization, olefin metathesis for on demand production of scarce propylene, conversion of methane to liquid aromatic fuels, biomass pyrolysis, water-gas shift for production of hydrogen and photocatalytic splitting of water to clean hydrogen.
The research aims to identify the catalytic active sites present on the heterogeneous catalyst surface to allow establishment of fundamental structure-activity/selectivity relationships that will guide the rational design of advanced catalysts. The research approach taken by the Wachs group is to simultaneously monitor the surface of the catalyst with spectroscopy under reaction conditions and online analysis of reactant conversion and product selectivity with online GC/mass spectrometer analysis. This new methodology has been termed operando spectroscopy and is allowing for the unprecedented development of molecular level structure-activity/selectivity relationships for catalysts. The spectroscopic techniques employed by the Wachs group for determination of the catalytic active sites and surface reaction intermediates are Raman, infrared (IR), ultra violet- visible (UV-vis), Near Atmospheric Pressure – X-ray Photoelectron Spectroscopy (NAP-XPS), High Sensitivity – Low Energy Ion Scattering (HS-LEIS), X-ray Absorption Spectroscopy (XANES/EXAFS), Nuclear Magnetic Resonance (NMR), Electron Paramagnetic Resonance (EPR), environmental transmission electron microscopy (STEM), Temperature Programmed Surface Reaction (TPSR) and Modulation Excitation Spectroscopy (MES). Isotopic labeling of Deuterium, Oxygen-18 and Carbon-13 is also used to track reaction pathways, determine rate-determining-steps and distinguish between spectator species and actual surface reaction intermediates.
The U.S. Environmental Protection Agency has honored Wachs with a Clean Air Excellence award for a catalytic process he invented that converts paper-mill pollutants into value-added formaldehyde. The American Chemical Society (ACS) has given Wachs the George A. Olah Award for achievements in hydrocarbon and petroleum chemistry and the American Institute of Chemical Engineering (AIChE) has honored Wachs with the Catalysis and Reaction Engineering Division Practice Award and R.H. Wilhelm Award in Chemical Reaction Engineering. He is the recipient of multiple awards from local catalysis societies (Michigan, New York, Chicago and Philadelphia). In 2011, he was named a Fellow of the American Chemical Society (ACS), the highest honor bestowed by the society. In 2012, he was recognized by the German Alexander von Humboldt Foundation with a Humboldt Research Award and the Vanadis award from the International Vanadium Chemistry Organization. More recently, he has been honored with the Lee Hsun Research Award in Materials Science, Institute Metal Research, Chinese Academy of Sciences (2015), Google Scholar ISI Golden 100 (2019) elected as a Fellow of the National Academy of Inventors (2019).
Wachs has published more than 300 highly cited technical articles (cited more than 40,000 times with a H index of 118) and holds more than three dozen U.S. patents. Additional details about the Wachs group activities (publications, presentations, awards, etc.) can be found on the group site (lehigh.edu/operando).
Related Links
- Nature Catalysis: “Induced activation of the commercial Cu/ZnO/Al2O3 catalyst for the steam reforming of methanol”
- Operando Molecular Spectroscopy and Catalysis Research Lab
- Faculty profile: Israel E. Wachs, Lehigh University
- U.S. Department of Energy, Office of Basic Energy Sciences
- National Natural Science Foundation of China
- ECUST: Minghui Zhu
- Lehigh University: Department of Chemical and Biomolecular Engineering
- Lehigh University: P.C. Rossin College of Engineering and Applied Science
Journal: Nature Catalysis
Article Title: Induced activation of the commercial Cu/ZnO/Al2O3 catalyst for the steam reforming of methanol
Article Publication Date: 20-Jan-2022
Media Contact
Katie Kackenmeister
Lehigh University
engineering@lehigh.edu
Office: 610-758-3632
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Silicon Carbide Innovation Alliance to drive industrial-scale semiconductor work
Known for its ability to withstand extreme environments and high voltages, silicon carbide (SiC) is a semiconducting material made up of silicon and carbon atoms arranged into crystals that is…

New SPECT/CT technique shows impressive biomarker identification
…offers increased access for prostate cancer patients. A novel SPECT/CT acquisition method can accurately detect radiopharmaceutical biodistribution in a convenient manner for prostate cancer patients, opening the door for more…

How 3D printers can give robots a soft touch
Soft skin coverings and touch sensors have emerged as a promising feature for robots that are both safer and more intuitive for human interaction, but they are expensive and difficult…





















