Role identified for key gene in developmental disability syndrome
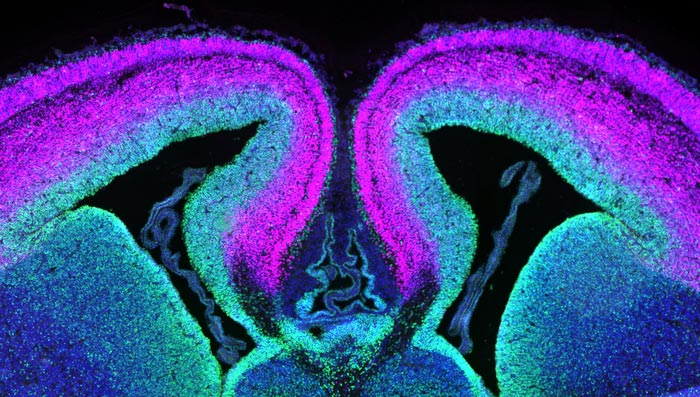
A cross section of mouse cerebral cortex with progenitor cells labeled green and neurons in magenta. Duke researchers have identified the role of a gene called DDX3X in the formation of healthy neurons.
Credit: Debra Silver Lab, Duke University
Single gene previously linked to rare syndrome of epilepsy, autism and developmental disability.
A single gene that was previously found to be the driving force in a rare syndrome linked to epilepsy, autism and developmental disability has been identified as a linchpin in the formation of healthy neurons.
Duke researchers say the gene, DDX3X, forms a cellular machine called a helicase, whose job it is to split open the hairpins and cul-de-sacs of RNA so that its code can be read by the protein-making machinery of the cell. This gene is carried on the X chromosome, so females have two copies of the gene and males have only one.
“If you remove both copies of the gene in a female mouse, that results in a massive microcephaly where the brains are severely reduced in size,” said Debra Silver, PhD, an associate professor of molecular genetics and microbiology in the Duke School of Medicine who led the research team. “But the removal of a single copy is probably more closely mimicking what’s happening in human patients,” Silver said.
Put another way, the defects caused by faulty DDX3X are dosage-dependent –- the syndrome can vary depending on how badly the production of helicases is affected by mutations. The findings appear June 28 in the open access journal eLife.
When DDX3X is altered by a mutation in early development, “you don’t get as many neurons over time because this gene is required for the production of neurons from progenitor cells,” Silver said. “And it is also helping the progenitors to divide properly.”
If it normally takes a nerve precursor cell 15 hours or so to divide, a mutated DDX3X may make that process take even longer, Silver said. “And what that means over time, if these neural precursors are taking too long to divide, is you fall behind, and the brain doesn’t develop properly.”
In a previous study the team published in March 2020, , using genetic samples from 107 developmentally disabled children from around the world, the researchers found that half of the DDX3X mutations disrupted the gene completely, but the other half only made it work more poorly.
DDX3X mutations are now considered the cause of 1 to 3 percent of intellectual disabilities in females, but the mutations are almost always ‘de novo,’ meaning they happened spontaneously during a developmental stage, rather than being inherited from the parents.
The children in the earlier study were almost all female, leading researchers to suppose that loss of DDX3X in males would be fatal, since they carry only a single copy of the gene. But in this work, Silver’s team discovered that a companion gene carried by the male’s Y chromosome, DDX3Y, can fulfill some of the gene’s function.
To do this work, Silver’s lab, led by Mariah Hoye, developed a new approach to profiling all the newly made proteins of progenitor cells in a living animal’s brain, a technique that could lead to an important understanding of protein synthesis in the brain, she said.
Some of the RNAs that have their translation reduced by damage to DDX3X also have roles in brain development, Silver said. “So it’s helping us to discover what I would call a network of RNAs whose translation depends on this gene. And it starts to give us clues as to how, molecularly, DDX3X may be disrupting brain development.”
DDX3X has also been implicated in neurodegeneration, some cancer progression and innate immune responses. Silver said understanding the cellular processes and molecular targets of DDX3X in the developing brain may help shed light on the basis for many disorders.
“We know of more than 800 families worldwide who have been diagnosed with DDX3X syndrome,” Silver said. “This is definitely an important gene, with likely hundreds of mutations. There’s really tons to learn about how DDX3X controls brain development.”
“We hope this research can improve an understanding of the basis for DDX3X syndrome and related disorders,” Silver said. “In the longer-term this may help contribute to development of therapies.”
Funding for this study was received from the DDX3X Foundation, the Holland-Trice Foundation, the Pew Charitable Trusts, and the National Institutes of Health (R21-ND104514, R01-NS120667, F32-NS112566)
CITATION: “Aberrant Cortical Development is Driven by Impaired Cell Cycle and Translational Control in a DDX3X Syndrome Model,” Mariah L. Hoye, Lorenzo Calviello, Abigail J. Poff, Nna-Emeka Ejimogu, Carly R. Newman, Maya D. Montgomery, Jianhong Ou, Stephen N. Floor, Debra L. Silver. eLife, June 28, 2022. DOI: 10.7554/eLife.78203
Online (Open Access): https://elifesciences.org/articles/78203
Journal: eLife
DOI: 10.7554/eLife.78203
Method of Research: Experimental study
Subject of Research: Animals
Article Title: Aberrant Cortical Development is Driven by Impaired Cell Cycle and Translational Control in a DDX3X Syndrome Model
Article Publication Date: 28-Jun-2022
Media Contact
Karl Bates
Duke University
karl.bates@duke.edu
Office: 919-681-8054
Original Source
https://today.duke.edu/2022/06/role-identified-key-gene-developmental-disability-syndrome
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…





















