Additional Mechanism Regulates Protein Activity

A University of Arkansas researcher and his colleagues have discovered a new mechanism that regulates the interaction of proteins in cell membranes. This discovery may lead to more efficient drug screening and possibly different methods for fighting infections.
Roger Koeppe, University Professor of chemistry and biochemistry, Thomas Suchnya, Frederick Sachs and Phillip Gottlieb of SUNY Buffalo and Sonya Tape and Olaf Andersen of Weil Medical College of Cornell University report their findings in the July 8, 2004, issue of Nature.
Scientists have explained the interaction of antibiotics using a “lock and key,” model, where a small drug of a certain shape (the key) binds to a bacterial protein (the lock) to neutralize it and prevent the spread of an infection.
In the Nature paper, the researchers show that this model is not the only rule in drug-protein interaction. They discovered that the mirror image of a peptide isolated from tarantula venom had the same effect on a certain type of pressure-sensitive cell membrane protein channel as did the natural peptide toxin – a finding that violates the “lock and key” model because the toxin and its mirror image have different shapes.
Further, they found that the mirror images of bacterial gramicidin channels, developed in the Koeppe laboratory at the University of Arkansas, respond much like natural gramicidin channels to both the tarantula toxin and its mirror image
“The effect is similar in different chemical systems,” Koeppe said. The researchers have concluded that, instead of working by the traditional “lock and key” model, the peptide toxin and its mirror image change the shape or curvature of the lipid bilayer, or the protective “skin” of the cell membrane.
This finding opens up a host of new applications, including the possibility of using mirror image proteins for drug therapies. Often, the mirror image peptides or proteins are biologically more stable and, if developed into drugs, could last longer in the body, Koeppe said. Also, the mirror image proteins don’t activate the body’s immune system as effectively, which could have a positive impact on organ transplant acceptance.
The gramicidin channel system also could be used to screen the generalized effects of potential drugs on the mechanical properties of lipid bilayer membranes.
“When a company develops a drug, they usually only want it to affect one thing,” Koeppe said. If a drug alters cell membrane properties globally, then its effects may prove too general, he said.
“If new drugs could be tested on gramicidin channels, it could speed up predictions of what such drugs would do in other systems,” Koeppe said. The cell membrane effects may be desirable or undesirable depending upon the system. “This could help companies find out early if there is a problem instead of investing three years and then finding out.”
Media Contact
More Information:
http://www.uark.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles
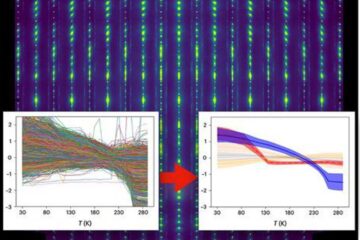
Machine learning algorithm reveals long-theorized glass phase in crystal
Scientists have found evidence of an elusive, glassy phase of matter that emerges when a crystal’s perfect internal pattern is disrupted. X-ray technology and machine learning converge to shed light…
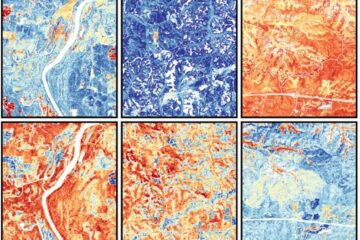
Mapping plant functional diversity from space
HKU ecologists revolutionize ecosystem monitoring with novel field-satellite integration. An international team of researchers, led by Professor Jin WU from the School of Biological Sciences at The University of Hong…

Inverters with constant full load capability
…enable an increase in the performance of electric drives. Overheating components significantly limit the performance of drivetrains in electric vehicles. Inverters in particular are subject to a high thermal load,…





















