Polarization of Br2 molecule in vanadium oxide cluster cavity and new alkane bromination
Peak of the spectrum due to polarized bromine molecule Credit: Kanazawa University
The bromine molecule (Br2) is widely used for bromination of a variety of organic compounds, where bromination reactions take place via a radical mechanism*2).
To attain product selectivity different from that of the radical mechanism, control of electron states of bromine molecule is needed.
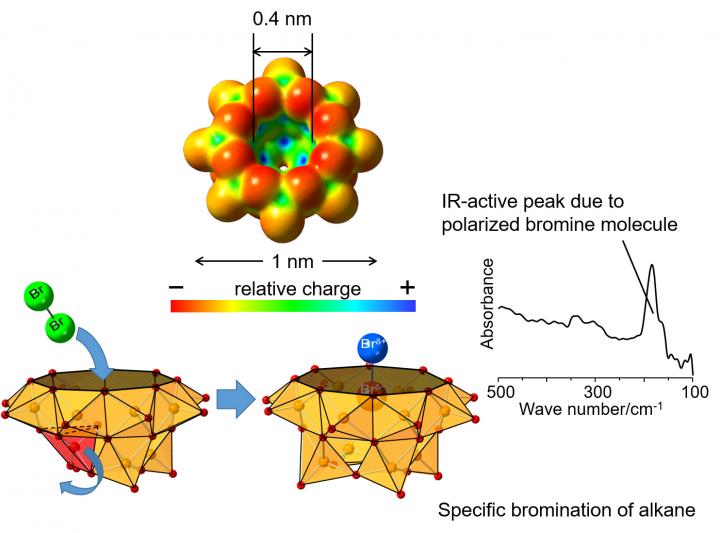
Vanadium oxide clusters*3) are a group of materials with various structures that are expected to be useful as functional materials. A hemispherical vanadium oxide cluster having a cavity corresponding to the size of one halogen atom shows special charge distribution where the periphery of the cavity is relatively negatively charged while the inside is relatively positively charged.
Although this compound has a large negative charge, it offers stable accommodation of a compound with a negative charge or with functional groups in its cavity.
Prof. Yuji Kikukawa of Kanazawa University previously revealed that the hemispherical vanadium oxide cluster took on a bulged structure in the presence of another compound being trapped in the cavity, whereas the structure was collapsed in the absence of a compound in the cavity (Angewandte Chemie, International Edition, 2018, 57, 16051-16055).
[Results]
In the present study led by a research group of Profs. Yuji Kikukawa and Yoshihito Hayashi of Kanazawa University in collaboration with scientists from Ritsumeikan University and High Energy Accelerator Research Organization, it was revealed that a bromine molecule can be stabilized in the cavity of a hemispherical vanadium oxide cluster.
In the infrared spectrum, an absorption peak at 185 cm-1 originating from polarization*4) of the bromine molecule trapped in the cavity was observed, although a bromine molecule without polarization would not show such a peak. This is the first spectral o
bservation of the polarized bromine molecule. By analyzing extended X-ray absorption fine structure measurements of the bromine molecule performed at Photon Factory, High Energy Accelerator Research Organization (KEK), a Br-Br distance of 0.233 nm was suggested, slightly longer than that of 0.228 nm in gas-phase bromine molecules.
By using such a polarized and activated bromine molecule in the cavity of the vanadium oxide cluster, bromination of pentane yielded 2-bromopentane and 3-bromopentane in a ratio of 36:64, which differs from the ratio of 80:20 when bromination was carried out in the absence of vanadium oxide cluster, indicating different selectivity.
In addition, concerning another product, 2,3-dibromopentane, which consists of diastereomers*5), the ratio of the threo isomer was higher than when bromine molecules alone were reacted with pentane. Furthermore, bromination could take place with smaller alkanes with shorter carbon chain such as butane or propane.
As above, it was found that the bromine molecule trapped in the vanadium oxide cavity showed a specificity different from the radical mechanism for bromination reaction of alkanes.
[Future prospects]
Metal oxide clusters are able to perform oxidization and reduction while maintaining their structure. It is also possible to conjugate with other metal species and to replace some constituent metal atoms with other atoms.
Thus, the characteristics of metal oxide clusters can be regulated. Further developments are expected such as the activation of small molecules using such an atomic dimension cavity through controlling charge distribution in the cavity and the production of highly functional catalysts by controlling molecular-level structures.
It is also expected that selective functionalization reactions using methane, which is highly inert but whose efficient chemical modification is highly desirable, can be attained by improving materials that regulate the electron states.
###
[Glossary]
*1) Functionalization
Atomic groups and bonds that characterize the properties and reactions of organic compounds.
*2) Radical mechanism
A mechanism, among chemical reaction mechanisms, that proceeds with atoms and molecules having unpaired electrons. In the case of bromine, the reaction mechanism in which bromine atoms are involved.
*3) Vanadium oxide cluster
Negatively charged molecular vanadium oxide.
*4) Polarization
A state in which the density of electrons on atoms is not equivalent but is asymmetrically distributed between the two directly bonded atoms.
*5) Diastereomers
Stereoisomers of chemical substances that are not mirror-image isomers (enantiomers). Diastereomers with two adjacent asymmetric carbons are called threo and erythro isomers and are distinguished.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Making diamonds at ambient pressure
Scientists develop novel liquid metal alloy system to synthesize diamond under moderate conditions. Did you know that 99% of synthetic diamonds are currently produced using high-pressure and high-temperature (HPHT) methods?[2]…

Eruption of mega-magnetic star lights up nearby galaxy
Thanks to ESA satellites, an international team including UNIGE researchers has detected a giant eruption coming from a magnetar, an extremely magnetic neutron star. While ESA’s satellite INTEGRAL was observing…

Solving the riddle of the sphingolipids in coronary artery disease
Weill Cornell Medicine investigators have uncovered a way to unleash in blood vessels the protective effects of a type of fat-related molecule known as a sphingolipid, suggesting a promising new…





















