Migrating holes help catalysts be productive
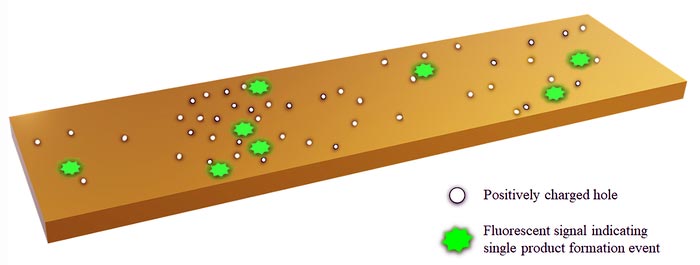
Positively charged holes that propagate at catalytic sites can spread out and trigger catalysis in neighboring sectors, according to a theory developed at Rice University and the Indian Institute of Science Education and Research, Pune.
Credit: Illustration by Bhawakshi Punia and Srabanti Chaudhury/IISER Pune
Theory shows how manipulating quasiparticles could improve chemical processes.
Dig this: Catalysis appears to function because of holes.
They’re not the physical kind. These quasiparticles, also known as electron holes, can be viewed as effective positive particles in the absence of negative electrons. And according to Rice University researchers, their action and propagation on heterogeneous catalyst particles allows the chemical reactions to happen.
The Rice lab of chemist Anatoly Kolomeisky and his colleague Professor Srabanti Chaudhury at the Indian Institute of Science Education and Research (IISER) Pune found through computational models that they might gain greater control over catalytic reactions by applying electric fields that prompt holes to migrate. That could lead to improvements in the ubiquitous process.
“Despite the fact that we’ve known about catalysis for 200 years — it goes back to cellars in the 19th century — we still don’t understand what’s happening on a catalyst,” said Kolomeisky, a professor and chair of Rice’s Department of Chemistry and a professor of chemical and biomolecular engineering. “But a couple of years ago I saw a series of intriguing papers from people at Cornell who were able to see co-catalytic cooperation involving positively charged particles.”
The Rice and IISER researchers set out to explain what the Cornell team saw, and found their theory matched the experiments. Their models show that holes are produced when a catalytic site activates and they then spread to adjacent sites, activating neighboring sites as well before recombining with electrons and dying.
The paper by Kolomeisky, Chaudhury and Bhawakshi Punia of IISER Pune appears in the Proceedings of the National Academy of Sciences.
Kolomeisky said the game-changing innovation by the Cornell team was in making the product of the catalyzed reactions they investigated to be fluorescent, so they could track them over time and space. “They realized to their surprise that if a reaction happens in one segment of a nanorod, it’s much more probable for the same reaction to happen on an adjacent segment. This cooperativity weakens down with distance, but it’s still surprising,” he said.
The new work suggests that each catalytic site on a nanorod probably makes positively charged holes during the redox chemical reaction that involves transfer of charged particles like electrons. As the local concentration of holes increases, they want to move to less-crowded areas. Where they settle, they trigger more catalysis and then die. The new catalytic site then makes more holes that further spread into neighboring regions.
“Our model is still highly speculative, because there is a clearly very complex underlying quantum mechanical phenomena here, and we don’t know what it is,” said Kolomeisky. “But our models quantitatively match the experiments. This should stimulate more investigations of these intriguing phenomena.
“If our theoretical predictions are valid, it will be possible to create an artificial density of holes in a controllable way, or create structures where holes might diffuse faster, and it might open a lot of channels for applications,” he said. “We might design new catalysts in the most efficient way.”
The Welch Foundation (C-1559), the National Science Foundation (1953453, 1941106), the NSF-supported Center for Theoretical Biological Physics at Rice (2019745), the Prime Minister Research Fellowship and an IISER Pune IDEAS Scholarship supported the research.
This news release can be found online at https://news.rice.edu/news/2022/migrating-holes-help-catalysts-be-productive.
Follow Rice News and Media Relations via Twitter @RiceUNews.
Related materials:
Kolomeisky Research Group: http://python.rice.edu/~kolomeisky/
Department of Chemistry: https://chemistry.rice.edu
Wiess School of Natural Sciences: https://naturalsciences.rice.edu
Image for download:
https://news-network.rice.edu/news/files/2021/12/0110_NANO-1-WEB.jpg
Positively charged holes that propagate at catalytic sites can spread out and trigger catalysis in neighboring sectors, according to a theory developed at Rice University and the Indian Institute of Science Education and Research, Pune. (Credit: Illustration by Bhawakshi Punia and Srabanti Chaudhury/IISER Pune)
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation’s top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 4,052 undergraduates and 3,484 graduate students, Rice’s undergraduate student-to-faculty ratio is just under 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is ranked No. 1 for lots of race/class interaction and No. 1 for quality of life by the Princeton Review. Rice is also rated as a best value among private universities by Kiplinger’s Personal Finance.
Journal: Proceedings of the National Academy of Sciences
DOI: 10.1073/pnas.2115135119
Article Title: Microscopic mechanisms of cooperative communications within single nanocatalysts
Article Publication Date: 10-Jan-2022
COI Statement: None
Media Contacts
Mike Williams
Rice University
mikewilliams@rice.edu
Office: 713-348-6728
Jeff Falk
Rice University
jfalk@rice.edu
Office: 713-348-6775
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Bringing bio-inspired robots to life
Nebraska researcher Eric Markvicka gets NSF CAREER Award to pursue manufacture of novel materials for soft robotics and stretchable electronics. Engineers are increasingly eager to develop robots that mimic the…

Bella moths use poison to attract mates
Scientists are closer to finding out how. Pyrrolizidine alkaloids are as bitter and toxic as they are hard to pronounce. They’re produced by several different types of plants and are…

AI tool creates ‘synthetic’ images of cells
…for enhanced microscopy analysis. Observing individual cells through microscopes can reveal a range of important cell biological phenomena that frequently play a role in human diseases, but the process of…





















