Lighting the way for nanotube innovation
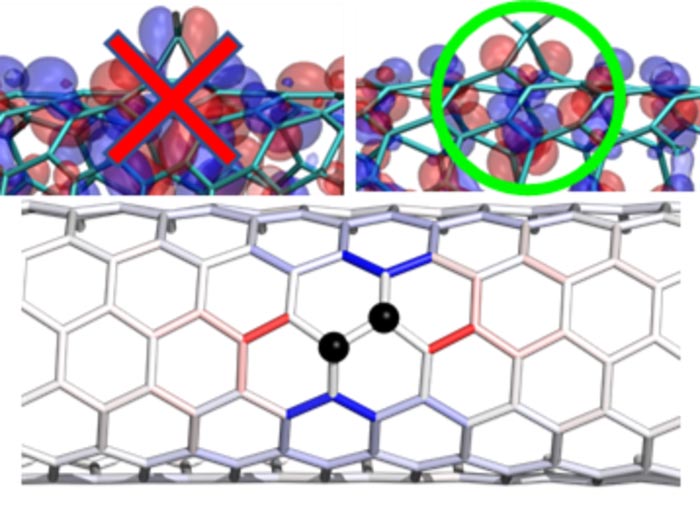
The introduction of controlled divalent bonds stabilizes the creation of potential energy wells (top), particularly along specific carbon atoms structures on the nanotube surface (bottom).
Credit: Image reprinted, with permission, from Brendan J. Gifford, et al. “Optical Effects of Divalent Functionalization of Carbon Nanotubes.” Chemistry of Materials 31, 6950. Copyright 2019 American Chemical Society.
Nanotubes with designed defects allow better performance for next-generation optical telecommunications.
The Science
Scientists have learned how to place crystalline defects in new materials with atomic-scale precision. This enables materials that can control excitons—energy carriers that are similar to subatomic particles. New research shows that, by precisely attaching specific chemical compounds to a carbon nanotube surface, scientists can create local energy wells that “capture” the excitons. The wells lower the excitons’ energy state. This prevents the loss of their energy as heat and controls the color of the light that they emit.
The Impact
Small but profound improvements drive every generation of breakthroughs in optical telecommunications. New component materials allow devices to be smaller, more efficient, and more accurate. However, these materials work best when researchers design and build them from nanoscale building blocks. These tiny building blocks are only billionths of a meter wide. These materials offer brighter, more controlled light emission that is closer to the infrared spectrum required for telecommunications.
Summary
Nanotubes are hollow cylinders of hexagonally bonded carbon sheets that are only one atom thick. Their electrical, elastic, thermal, and optical properties are particularly interesting for advanced telecommunications materials. The challenge has been that single-walled carbon nanotubes tend to emit light inefficiently and at the less-useful blue end of the light wave spectrum. These factors make them less suitable for telecommunications. The inefficiency stems from the rapid movement of excited electrons (or “excitons”) across the surface of the nanotubes. These excitons decay and lose their energy as heat when they encounter natural structural defects on the surface. Optically useful excited nanotubes must therefore minimize the production of heat, maximize light emission, and produce light closer to the infrared telecommunication-relevant spectrum. Attaching specific chemical groups to the surface of the nanotube modifies the potential energy landscape by creating “energy wells” along the surface of the nanotube. The wells attract the free-floating surface excitons and trap them in areas a few nanometers long. Because the excited electrons cannot move freely, they are “forced” to release energy as light rather than heat. The trapped excitons also have a lower energy state, which “redshifts” the emitted light waves closer to the desired infrared part of the spectrum.
In this study, scientists from the Center for Integrated Nanotechnologies, a Department of Energy (DOE) Office of Science user facility, and their co-authors tested three new types of chemical groups on single-walled carbon nanotubes. The researchers created theoretical models of atomic-scale structures that optimized the placement of stable chemical bonds to maximize the optical emission of the nanotubes. They verified the results experimentally, providing direct evidence that the modified surfaces improved light emission. This innovation will help future teams to create more finely tuned optical functions in chemically modified nanotubes.
Funding
This work was conducted in part at the Center for Integrated Nanotechnologies, a DOE Office of Science user facility, and the Los Alamos National Laboratory (LANL) Center for Nonlinear Studies. Research by individual co-authors was supported in part by the LANL Laboratory Directed Research and Development program, the National Science Foundation, the Alfred P. Sloan Research Fellowship, the Center for Computationally Assisted Science and Technology at North Dakota State University, the National Energy Research Scientific Computing Center, and the LANL Institutional Computing Program.
Journal: Chemistry of Materials
DOI: 10.1021/acs.chemmater.9b01438
Method of Research: Experimental study
Subject of Research: Not applicable
Article Title: Optical Effects of Divalent Functionalization of Carbon Nanotubes
Article Publication Date: 16-May-2019
Media Contact
Michael Church
michael.church@science.doe.gov
Office: 505-358-1481
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

“Nanostitches” enable lighter and tougher composite materials
In research that may lead to next-generation airplanes and spacecraft, MIT engineers used carbon nanotubes to prevent cracking in multilayered composites. To save on fuel and reduce aircraft emissions, engineers…

Trash to treasure
Researchers turn metal waste into catalyst for hydrogen. Scientists have found a way to transform metal waste into a highly efficient catalyst to make hydrogen from water, a discovery that…

Real-time detection of infectious disease viruses
… by searching for molecular fingerprinting. A research team consisting of Professor Kyoung-Duck Park and Taeyoung Moon and Huitae Joo, PhD candidates, from the Department of Physics at Pohang University…





















