Discovery of a new catalytic intermediate in biological water oxidation
EPR-Spektroskopie und quantenchemische Untersuchung des Wasseroxidationskatalysators der biologischen Photosynthese
Dr. Dimitrios Pantazis, MPI für Kohlenforschung
Computational simulations performed by the Pantazis group combined with spectroscopic studies led to the discovery of a new catalytic intermediate in water oxidation.
One of the greatest ambitions of modern chemistry is to reproduce with synthetic catalysts the remarkable feat of water oxidation performed in nature by photosynthetic organisms. Plants use sunlight to split water into oxygen, protons, and electrons. This is the source of the oxygen that we breathe, while the protons and electrons are used in the enzymatic reactions that incorporate atmospheric carbon dioxide into biomolecules. Despite decades of dedicated research efforts, many details of nature’s process remain shrouded in mystery. Very recently, a significant step towards deciphering the mechanism of water oxidation by plants was made by an international team of researchers led by Dr. Dimitrios Pantazis, group leader at the Max-Planck-Institut für Kohlenforschung.
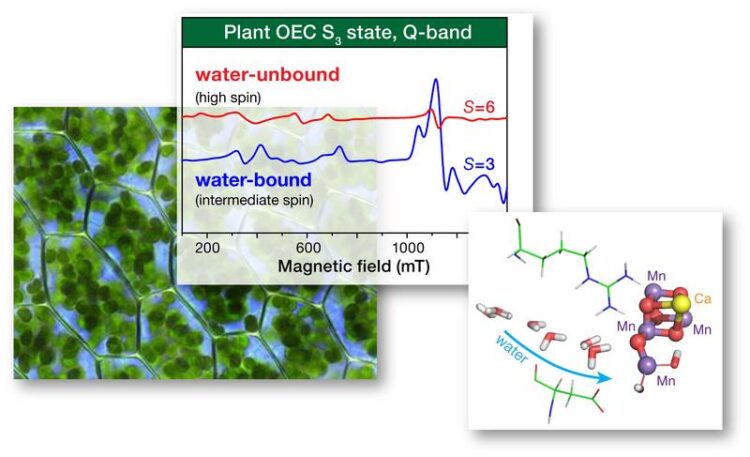
Guided by spectroscopic studies and computational simulations performed by the Pantazis group during the past few years, the research team designed a series of experiments that intended to inhibit the binding of water at the active site of the enzyme responsible for water oxidation, Photosystem II.
Using methanol as an inhibitor to block a protein channel that delivers water to the catalytic site, it was possible to isolate and study a previously unknown form of the catalyst, the intermediate that is responsible for binding and activating the water molecule for subsequent oxidation. Using electron paramagnetic resonance (EPR) spectroscopy in combination with advanced quantum chemical techniques, this previously unknown intermediate state was shown to contain a manganese ion with a very unusual coordination geometry that has no exact precedence in synthetic chemistry.
This manganese ion has only five instead of the normal six ligands and this creates an important free coordination site where water can bind. The discovery of the new catalytic intermediate makes it necessary to reconsider the hypothetical reaction mechanisms discussed so far for water oxidation in plants, but it also has important implications for the design and operational principles of artificial water splitting systems.
The study is published as a “Hot Paper” in the prestigious journal Angewandte Chemie.
Wissenschaftliche Ansprechpartner:
Dr. Dimitrios Pantazis, Group Leader at the Max-Planck-Institut für Kohlenforschung
Phone: +49 208 306 2156
Originalpublikation:
G. Zahariou, N. Ioannidis, Y. Sanakis, D. A. Pantazis. “Arrested Substrate Binding Resolves Catalytic Intermediates in Higher‐Plant Water Oxidation”, Angewandte Chemie International Edition. https://onlinelibrary.wiley.com/doi/10.1002/anie.202012304
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…





















