Discovery adapts natural membrane to make hydrogen fuel from water
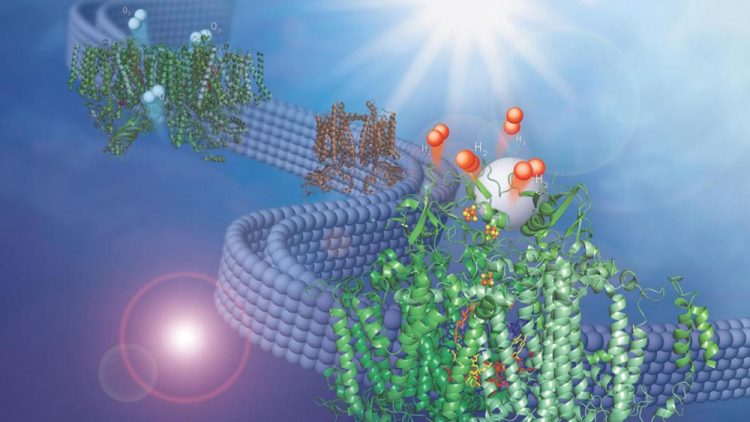
This image shows two membrane-bound protein complexes that work together with a synthetic catalyst to produce hydrogen from water. Credit: Olivia Johnson and Lisa Utschig
In a recent study from the U.S. Department of Energy's (DOE) Argonne National Laboratory, scientists have combined two membrane-bound protein complexes to perform a complete conversion of water molecules to hydrogen and oxygen.
The work builds on an earlier study that examined one of these protein complexes, called Photosystem I, a membrane protein that can use energy from light to feed electrons to an inorganic catalyst that makes hydrogen. This part of the reaction, however, represents only half of the overall process needed for hydrogen generation.
By using a second protein complex that uses energy from light to split water and take electrons from it, called Photosystem II, Argonne chemist Lisa Utschig and her colleagues were able to take electrons from water and feed them to Photosystem I.
“The beauty of this design is in its simplicity — you can self-assemble the catalyst with the natural membrane to do the chemistry you want” — Lisa Utschig, Argonne chemist
In an earlier experiment, the researchers provided Photosystem I with electrons from a sacrificial electron donor. “The trick was how to get two electrons to the catalyst in fast succession,” Utschig said.
The two protein complexes are embedded in thylakoid membranes, like those found inside the oxygen-creating chloroplasts in higher plants. “The membrane, which we have taken directly from nature, is essential for pairing the two photosystems,” Utschig said.
“It structurally supports both of them simultaneously and provides a direct pathway for inter-protein electron transfer, but doesn't impede catalyst binding to Photosystem I.”
According to Utschig, the Z-scheme — which is the technical name for the light-triggered electron transport chain of natural photosynthesis that occurs in the thylakoid membrane — and the synthetic catalyst come together quite elegantly. “The beauty of this design is in its simplicity — you can self-assemble the catalyst with the natural membrane to do the chemistry you want,” she said.
One additional improvement involved the substitution of cobalt or nickel-containing catalysts for the expensive platinum catalyst that had been used in the earlier study. The new cobalt or nickel catalysts could dramatically reduce potential costs.
The next step for the research, according to Utschig, involves incorporating the membrane-bound Z-scheme into a living system. “Once we have an in vivo system — one in which the process is happening in a living organism — we will really be able to see the rubber hitting the road in terms of hydrogen production,” she said.
###
A paper based on the research, “Z-scheme solar water splitting via self-assembly of photosystem I-catalyst hybrids in thylakoid membranes,” appeared in the October 29 online edition of Chemical Science. Other Argonne authors of the paper included Sarah Soltau, Karen Mulfort, Jens Niklas and Oleg Poluektov.
The research was funded by the DOE Office of Science, Basic Energy Sciences Program.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

A flexible and efficient DC power converter for sustainable-energy microgrids
A new DC-DC power converter is superior to previous designs and paves the way for more efficient, reliable and sustainable energy storage and conversion solutions. The Kobe University development can…
Technical Trials for Easing the (Cosmological) Tension
A new study sorts through models attempting to solve one of the major challenges of contemporary cosmic science, the measurement of its expansion. Thanks to the dizzying growth of cosmic…

Peptides on Interstellar Ice
A research team led by Dr Serge Krasnokutski from the Astrophysics Laboratory at the Max Planck Institute for Astronomy at the University of Jena had already demonstrated that simple peptides…





















