Coat of proteins makes viruses more infectious and links them to Alzheimer's disease
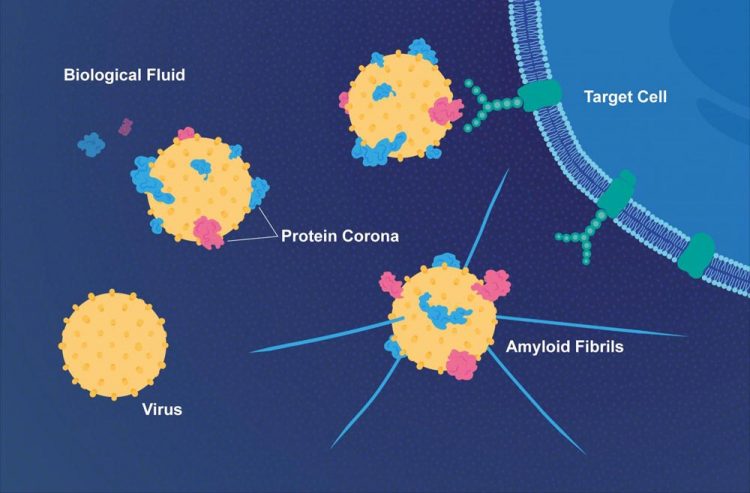
Proteins in the fluid surrounding the target cell bind to a virus and can make it more infectious. The virus can also accelerate the formation of threadlike amyloid fibrils that play a part in Alzheimer's disease. Credit: E. Wikander/Azote/Stockholm University Usage Restrictions: Image may be used in connection with news articles related to Stockholm University.
Are viruses dead or alive? Well… both. Viruses can only reproduce inside living cells and exploit the cellular machinery of their host to their benefit. However, before entering a host cell, viruses are just nanometer-sized particles, very similar to artificial nanoparticles used in medical applications for diagnosis and therapy.
Scientists from Stockholm University and Karolinska Institutet have found that viruses and nanoparticles share another important property; they both become covered by a layer of proteins when they encounter the biological fluids of their host before they find their target cell. This layer of proteins on the surface influence their biological activity significantly.
“Imagine a tennis ball falling into a bowl of milk and cereals. The ball is immediately covered by the sticky particles in the mix and they remain on the ball when you take it out of the bowl. The same thing happens when a virus gets in contact with blood or lung fluids that contain thousands of proteins. Many of these proteins immediately stick to the viral surface forming a so-called protein corona”, Kariem Ezzat of Stockholm University and Karolinska Institutet explains.
Kariem Ezzat and his colleagues studied the protein corona of respiratory syncytial virus (RSV) in different biological fluids. RSV is the most common cause of acute lower respiratory tract infections in young children worldwide, leading up to 34 million cases and 196,000 fatalities each year. “The protein corona signature of RSV in the blood is very different from that in lung fluids.
It is also different between humans and other species such as rhesus macaque monkeys, which also can be infected with RSV”, Kariem Ezzat says. “The virus remains unchanged on the genetic level. It just acquires different identities by accumulating different protein coronae on its surface depending on its environment. This makes it possible for the virus to use extracellular host factors for its benefit, and we've shown that many of these different coronae make RSV more infectious.”
The researchers from Stockholm University and Karolinska Institutet have also found that viruses such as RSV and herpes simplex virus type 1 (HSV-1) can bind a special class of proteins called amyloid proteins. Amyloid proteins aggregate into plaques that play a part in Alzheimer's disease where they lead to neuronal cell death.
The mechanism behind the connection between viruses and amyloid plaques has been hard to find till now, but Kariem Ezzat and his colleagues found that HSV-1 is able to accelerate the transformation of soluble amyloid proteins into thread-like structures that constitute the amyloid plaques. In animal models of Alzheimer's disease, they saw that mice developed the disease within 48 hours of infection in the brain. In absence of an HSV-1 infection the process normally takes several months.
“The novel mechanisms described in our paper can have an impact not only on understanding new factors determining how infectious a virus is, but also on devising new ways to design vaccines. In addition, describing a physical mechanism that links viral and amyloid causes of disease adds weight to the increasing research interest in the role of microbes in neurodegenerative disorders such as Alzheimer's disease and opens up new avenues for treatments.”, Kariem Ezzat of Stockholm University and Karolinska Institutet says.
###
The article
“The Viral Protein Corona Directs Viral Pathogenesis and Amyloid Aggregation” by Ezzat et al is published in Nature Communications. DOI: 10.1038/s41467-019-10192-2. The project is a collaboration between researcher from Stockholm University, Karolinska Institutet and University of Eastern Finland and others.
More information
Kariem Ezzat, Department of Molecular Biosciences, The Wenner-Gren Institute, Stockholm University and Department of Laboratory Medicine, Karolinska Institutet. Phone: + 46 8 16 14 37, mobile: +46 720 39 09 93, e-mail: kariem.ezzat@su.se
Anna-Lena Spetz, Department of Molecular Biosciences, The Wenner-Gren Institute, Stockholm University. Phone: +46 8 16 42 05, Mobile phone +46 707 47 13 03, e-mail: anna-lena.spetz@su.se
Media Contact
More Information:
http://dx.doi.org/10.1038/s41467-019-10192-2All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Silicon Carbide Innovation Alliance to drive industrial-scale semiconductor work
Known for its ability to withstand extreme environments and high voltages, silicon carbide (SiC) is a semiconducting material made up of silicon and carbon atoms arranged into crystals that is…

New SPECT/CT technique shows impressive biomarker identification
…offers increased access for prostate cancer patients. A novel SPECT/CT acquisition method can accurately detect radiopharmaceutical biodistribution in a convenient manner for prostate cancer patients, opening the door for more…

How 3D printers can give robots a soft touch
Soft skin coverings and touch sensors have emerged as a promising feature for robots that are both safer and more intuitive for human interaction, but they are expensive and difficult…





















