Binding of a second CO molecule observed
Bildunterschrift siehe Text. Grafik: Oliver Einsle
Through the biological fixation of the element nitrogen by the enzyme nitrogenase, organisms gain access to molecular nitrogen (N2) in the Earth‘s atmosphere, which is essential for building cellular structures. In addition, a vanadium-dependent variant of nitrogenase can reduce the toxic gas carbon monoxide (CO) to hydrocarbons. These reductions of N2 and CO are among the most important processes in industrial chemistry, as they are used to produce both fertilizers and synthetic fuels. However, researchers have not yet been able to decipher the different pathways of the two reactions.
Dr. Michael Rohde from Prof. Dr. Oliver Einsle’s team at the Institute of Biochemistry at the University of Freiburg, in collaboration with two research groups at Freie Universität Berlin, has now been able to show how the active site of the vanadium-dependent nitrogenase is able to bind two CO molecules simultaneously, thereby creating the basis for combining the spatially adjacent carbon atoms of both molecules in a reductive process. The researchers recently presented their results in the journal Science Advances.
The industrial reductions of N2 and CO – known as the Haber-Bosch and Fischer-Tropsch processes, respectively – require high temperatures and pressure. While N2 reduction leads to the bioavailable product ammonium (NH4+), at least two carbon atoms combine during the conversion of CO. The predominant reaction product is ethylene (ethene, C2H4), a colorless gas that plays an important role not only in fuels but also in the production of plastics. Although the cleavage of an N-N bond in nitrogen fixation is chemically quite fundamentally different from the formation of a C-C bond in CO reduction, scientists previously suspected that nitrogenase uses the same basic mechanistic principles for both reactions.
In a previous work, the team led by Rohde and Einsle used nitrogenase to react with CO gas, resulting in the specific binding of a single molecule. In their current study, which builds on this work, the researchers show that they gassed crystals of this first state with CO under pressure and then subjected them to X-ray crystallographic analysis. This allowed them to directly observe how a second CO molecule binds. “The form of nitrogenase obtained in this way, with two CO molecules at the active site, probably represents a blocked state,” Rohde explains, “but it provides direct clues to the mechanism of the enzyme.” As a result, Einsle’s team can now outline a detailed mechanism of CO reduction through nitrogenase.
Oliver Einsle serves as professor at the Institute of Biochemistry of the University of Freiburg and is a member of BIOSS Centre for Biological Signalling Studies of the University of Freiburg.
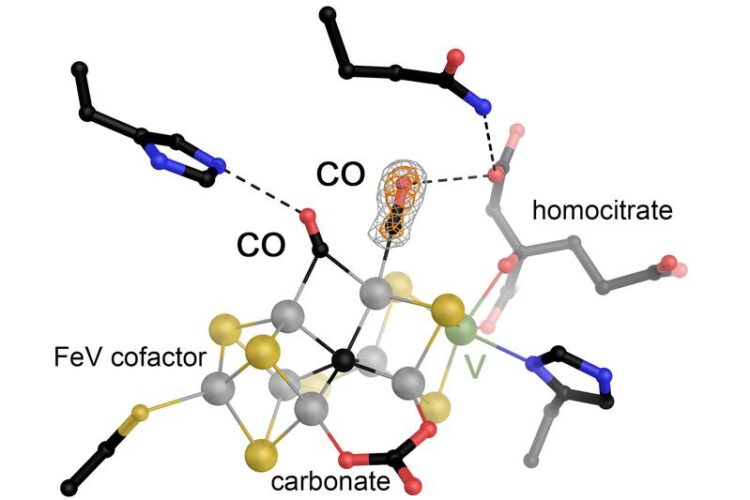
Image caption:
The iron-vanadium (FeV) cofactor in vanadium-dependent nitrogenase was made to react with carbon monoxide (CO) and then gassed under pressure, allowing two molecules of the substrate to be visualized in bound form. FeV cofactor is one of the largest and most complex metal centers in proteins currently known. It consists of seven iron ions (gray), 9 sulfur ions (yellow), a central carbon (black), and a vanadium ion (green), and also carries a carbonate ion and a molecule of homocitrate as organic ligands. Graphic: Oliver Einsle
Wissenschaftliche Ansprechpartner:
Prof. Dr. Oliver Einsle
Institute of Biochemistry / BIOSS Centre for Biological Studies
University of Freiburg
Tel.: 0761/203-6058
E-Mail: einsle@biochemie.uni-freiburg.de
Originalpublikation:
Rohde, M., Laun, K., Zebger, I., Stripp, S. T., Einsle, O. (2021): Two ligand binding sites in CO-reducing vanadium nitrogenase reveal a general mechanistic principle. In: Science Advances, Vol. 7, No. 22. DOI: 10.1126/sciadv.abg4474
Weitere Informationen:
https://www.pr.uni-freiburg.de/pm-en/press-releases-2021/binding-of-a-second-co-…
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Combatting disruptive ‘noise’ in quantum communication
In a significant milestone for quantum communication technology, an experiment has demonstrated how networks can be leveraged to combat disruptive ‘noise’ in quantum communications. The international effort led by researchers…

Stretchable quantum dot display
Intrinsically stretchable quantum dot-based light-emitting diodes achieved record-breaking performance. A team of South Korean scientists led by Professor KIM Dae-Hyeong of the Center for Nanoparticle Research within the Institute for…

Internet can achieve quantum speed with light saved as sound
Researchers at the University of Copenhagen’s Niels Bohr Institute have developed a new way to create quantum memory: A small drum can store data sent with light in its sonic…





















