Approved medications – new role in combating infections?
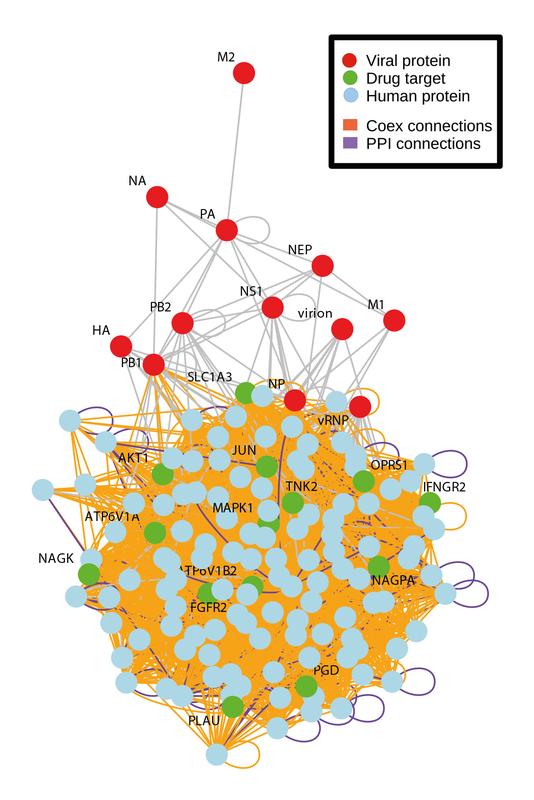
Using bioinformatics in search of effective medicinal products against influenza. Source: PEI
Influenza A virus ranks among the most feared pathogens. It regularly causes influenza epidemics. Its envelope contains the ion-channel forming M2 protein as well as the surface glycoproteins haemagglutinin (HA) and neurminidase (NA).
Based on the antigenic properties of these glycoproteins, the viruses are categorised into different subtypes. Currently, 18 different haemagglutinin and 11 neuramidase subtypes have been identified. Influenza vaccines contain antigen components of the different circulating subtypes. However, the high genetic variability of influenza viruses requires annual vaccine updates.
If a new influenza virus emerges, the development and production of a matching vaccine will take several months. During this time, compounds that block the virus (antivirals) are the only treatment option. However, resistance against these compounds – M2 protein ion channel blockers and neuraminidase inhibitors – rapidly emerges, reducing their efficacy.
Repurposing of compounds originally licensed for other indications to treat diseases like influenza is a promising new approach. Unlike compounds that are designed to target the pathogen directly, this approach looks for compounds that target proteins in the infected cell that are needed for the replication of the virus.
On the one hand, targeting cellular proteins is expected to be associated with less resistance development, and on the other hand, such a compound may be efficacious against a broad spectrum of virus strains.
So-called genome-wide screenings with “small interfering” RNAs (siRNAs) were used to identify candidate proteins and signaling pathways involved in the influenza virus replication cycle.
In co-operation with researchers from various different research institutes in Singapore, and with support from the German Centre for Infection Research (DZIF), Professor Veronika von Messling, head of Division Veterinary Medicine of the Paul-Ehrlich-Institut until September 2018, and her group have compared these candidates with known targets of already licensed compounds, which would be immediately available in the event of an epidemic.
The most promising candidates were then first tested in vitro (in cell culture) and followed by animal experiments with mice and ferrets in vivo.
Out of 15 candidate compounds, four were able to control an influenza A infection in vitro. In efficacy studies in mice, dextromethorphan led to a significant decrease in viral load in the lung and increased efficacy of the antiviral agent Oseltamivir. In ferrets infected with a seasonal H1N1 strain, the administration of dextromethorphan also reduced disease severity but not the virus titer in the lung.
“Our data show that dextromethorphan could be a therapeutic option for influenza”, said Professor von Messling. Furthermore, the study shows the potential of bioinformatics-based approaches to identify promising candidates for drug repurposing to treat infectious diseases.
The Paul-Ehrlich-Institut, the Federal Institute for Vaccines and Biomedicines, in Langen near Frankfurt/Main is a senior federal authority reporting to the Federal Ministry of Health (Bundesministerium für Gesundheit, BMG). It is responsible for the research, assessment, and marketing authorisation of biomedicines for human use and immunological veterinary medicinal products. Its remit also includes the authorisation of clinical trials and pharmacovigilance, i.e. recording and evaluation of potential adverse effects. Other duties of the institute include official batch control, scientific advice and inspections. In-house experimental research in the field of biomedicines and life science form an indispensable basis for the manifold tasks performed at the institute. The Paul-Ehrlich-Institut, with its roughly 800 members of staff, also has advisory functions nationally (federal government, federal states (Länder)), and internationally (World Health Organisation, European Medicines Agency, European Commission, Council of Europe etc.).
Enkirch T, Sauber S, Anderson DE, Gan ES, Kenanov D, Maurer-Stroh S, von Messling V (2018): Identification and In Vivo Efficacy Assessment of Approved Orally Bioavailable Human Host Protein-Targeting Drugs with Broad Anti-Influenza A Activity.
Front Immunol Jun 5 [Epub ahead of print].
DOI: 10.3389/fimmu.2019.01097
https://www.frontiersin.org/articles/10.3389/fimmu.2019.01097/abstract – Publication, Abstract
https://www.pei.de/EN/information/journalists-press/press-releases/2019/13-known… – This press release on the PEI Website
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Silicon Carbide Innovation Alliance to drive industrial-scale semiconductor work
Known for its ability to withstand extreme environments and high voltages, silicon carbide (SiC) is a semiconducting material made up of silicon and carbon atoms arranged into crystals that is…

New SPECT/CT technique shows impressive biomarker identification
…offers increased access for prostate cancer patients. A novel SPECT/CT acquisition method can accurately detect radiopharmaceutical biodistribution in a convenient manner for prostate cancer patients, opening the door for more…

How 3D printers can give robots a soft touch
Soft skin coverings and touch sensors have emerged as a promising feature for robots that are both safer and more intuitive for human interaction, but they are expensive and difficult…





















