New material for splitting water
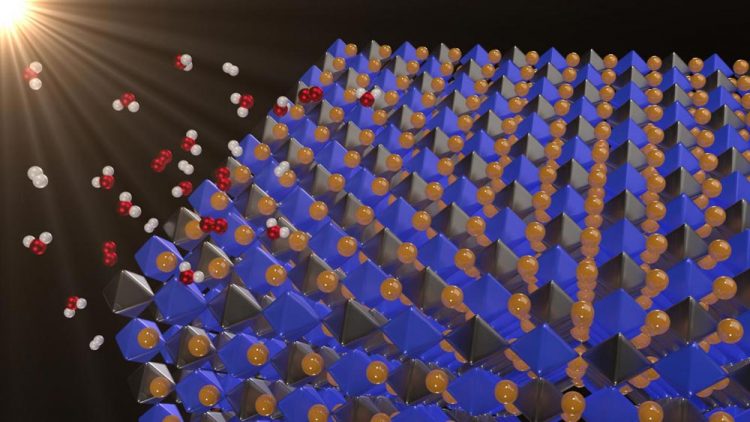
Solar energy is clean and abundant, but when the sun isn't shining, you must store the energy in batteries or through a process called photocatalysis. In photocatalytic water splitting, sunlight separates water into hydrogen and oxygen, which can then be recombined in a fuel cell to release energy. Now, a new class of materials -- halide double perovskites -- may have just the right properties to split water, according to research in Applied Physics Letters. In this image: Novel, lead-free double perovskites as potential photocatalysts for solar water splitting Credit: George Volonakis
Solar energy is clean and abundant. But when the sun isn't shining, you must store the energy in batteries or through a process called photocatalysis — in which solar energy is used to make fuels. In photocatalytic water splitting, sunlight separates water into hydrogen and oxygen. The hydrogen and oxygen can then be recombined in a fuel cell to release energy.
Now, a new class of materials — halide double perovskites — may have just the right properties to split water, according to a newly published paper in Applied Physics Letters, from AIP Publishing.
“If we can come up with a material that can be useful as a water-splitting photocatalyst, then it would be an enormous breakthrough,” said Feliciano Giustino, a co-author on the paper.
Researchers have experimented with many photocatalytic materials before, such as titanium dioxide (TiO2). While TiO2 can harness sunlight to split water, it's inefficient because it doesn't absorb visible light well. So far, no photocatalytic material for general water splitting has become commercially available.
Using supercomputers to calculate the quantum energy states of four halide double perovskites, George Volonakis and Giustino, both of the University of Oxford, found that Cs2BiAgCl6 and Cs2BiAgBr6 are promising photocatalytic materials because they absorb visible light much better than TiO2. They also generate electrons and holes (the positively charges absence of electrons) that have sufficient energy (or nearly ideal energies) to split water into hydrogen and oxygen.
Very few other materials have all these features at once, Giustino said. “We can't say this will work for sure, but these compounds seem to have all the right properties.”
Giustino and his team originally discovered this type of perovskite while looking for materials to make solar cells. Over the last several years, perovskites have garnered interest as materials to boost the efficiency of silicon-based solar cells through tandem designs that integrate a perovskite cell directly onto a high-efficiency silicon cell, but they contain a small amount of lead. If they were used for energy harvesting in a solar farm, the lead could pose a potential environmental hazard.
In 2016, using computer simulations to identify alternative materials, the researchers found a new type of lead-free perovskite with potential for high-efficiency solar cells. The present paper shows these new materials may also split water. “These new double perovskites are not only promising as a complementary material for tandem solar cells, but they can also be promising in areas like photocatalysis,” Volonakis said.
Still, the new analysis is theoretical, assuming the compounds form perfect crystals. The next step, the authors said, is for experimentalists to see if the material works in the real world as well as predicted. In the meantime, the researchers are using their computational techniques to explore whether these double perovskites have properties useful for other applications like light detectors.
###
The article, “Surface properties of lead-free halide double perovskites: Possible visible-light photo-catalysts for water splitting,” is authored by George Volonakis and Feliciano Giustino. The article appeared in Applied Physics Letters June 12, 2018, (DOI: 10.1063/1.5035274) and can be accessed at https:/
ABOUT THE JOURNAL
Applied Physics Letters features concise, rapid reports on significant new findings in applied physics. The journal covers new experimental and theoretical research on applications of physics phenomena related to all branches of science, engineering, and modern technology. See http://apl.
Media Contact
More Information:
http://dx.doi.org/10.1063/1.5035274All latest news from the category: Physics and Astronomy
This area deals with the fundamental laws and building blocks of nature and how they interact, the properties and the behavior of matter, and research into space and time and their structures.
innovations-report provides in-depth reports and articles on subjects such as astrophysics, laser technologies, nuclear, quantum, particle and solid-state physics, nanotechnologies, planetary research and findings (Mars, Venus) and developments related to the Hubble Telescope.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…





















