New 2-Color X-ray Laser Technique Could Reveal Atomic Detail of Medically Important Proteins
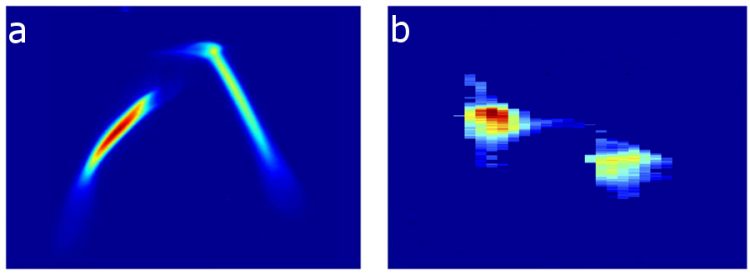
SLAC National Accelerator Laboratory These charts show (a) the energy profile of two electron bunches that are separated by about 6 picoseconds, or trillionths of a second. These electron bunches, produced by a specialized laser striking a copper device called a cathode, are compressed and later emit (b) two X-ray pulses of different energies separated by about 35 femtoseconds, or quadrillionths of a second, which are useful for a range of experiments at SLAC's Linac Coherent Light Source X-ray laser. The two X-ray pulses can have different wavelengths, or "colors," and hit samples either at the same time or with a delay between pulses up to 120 femtoseconds.
A unique X-ray laser innovation developed at the Department of Energy's SLAC National Accelerator Laboratory may make it easier and faster for scientists to fully map medically important proteins whose structures have remained stubbornly out of reach.
They include signaling proteins that are considered prime targets for drug design because they regulate our immune systems, moods, senses and other vital functions, as well as components of the body’s delicate protein-making machinery.
The innovation could also yield new information from hard-to-study samples in other fields, such as materials science and chemistry, in experiments at SLAC’s Linac Coherent Light Source (LCLS), a DOE Office of Science User Facility.
It involves hitting samples with pairs of precisely tuned X-ray laser pulses of different colors, or wavelengths. When the pulses hit at the same time, they can give scientists a more detailed 3-D view of the sample’s structure. When they hit one after the other – so the first pulse begins a reaction in the sample and the second captures an X-ray snapshot of the resulting changes – they can be used to study processes, such as the earliest steps of chemical reactions
To achieve this, researchers incorporated a series of recent X-ray laser advances that are described in separate papers published Dec. 18 in Physical Review Letters and March 6 in Nature Communications.
“This is one of the most important developments to date in X-ray lasers. We hope to make use of this in the effort to revolutionize protein structural analysis using X-ray lasers,” said Soichi Wakatsuki, a professor of photon science at SLAC and of structural biology at the Stanford School of Medicine. “A lot of work went into developing this, and we weren’t sure at first whether it could be done.”
Crystallography: Building on Success
One important application will be in a new generation of LCLS experiments that use X-ray crystallography to determine protein structures that are difficult or impossible to obtain with previous methods.
X-ray crystallography has revealed the structures of more than 100,000 proteins since it came into use at synchrotron X-ray facilities decades ago. In this technique, X-rays strike crystallized proteins and produce diffraction patterns that can be analyzed to reconstruct a 3-D atomic structure.
One inherent problem with the technique is that the X-rays do not directly produce an image of the proteins; like a camera with a bright flash but no lens, detectors capture the intensity of the light but no recognizable image.
Scientists working at synchrotrons can get around this so-called “phase problem” by adding metals to samples. The metals serve as location-finding beacons within the molecule that allow scientists to extract its full structure with complex analysis tools. Synchrotron researchers also hit crystallized proteins with X-rays of different colors to produce separate sets of diffraction patterns that together reveal the full 3-D structure – akin to slightly different, coupled images viewed through 3-D glasses.
Faster Structural Analysis
With its ultrashort X-ray laser pulses that are a billion times brighter than previous sources, LCLS can resolve the structures of proteins from crystals that are too small or fragile for study at synchrotrons, potentially opening tens of thousands of proteins to routine analysis.
Now that the two-color technique is also available at LCLS, Wakatsuki said, researchers will hopefully be able to analyze completely unknown protein structures more rapidly, and without reference to similar structures. This, coupled with the ability of LCLS to study samples in very natural conditions, will be especially important when using those structures to develop and screen potential candidates for new drugs.
Because LCLS X-ray pulses are so bright, making use of them for biological studies presents a technical challenge: In order for the new technique to succeed, the two X-ray pulses must hit crystals simultaneously – otherwise the first pulse will damage or destroy the sample before the second one arrives.
To best control the timing and properties of both pulses, SLAC scientists accelerated two electron bunches so they can emit X-rays of different colors with a peak power 10 times higher than previously possible. They also incorporated earlier LCLS innovations, including a system that produces a sharp spike in power in a predictable range of colors for each pulse, and a diagnostic tool that precisely measures the duration and overlap of the two X-ray pulses.
Timing Is Everything
“Before this we couldn’t independently control the color of the two pulses, nor could we achieve the simultaneous timing or a wide enough separation in energy,” said Alberto Lutman, a SLAC scientist and lead author of the Physical Review Letters paper.
“Now we have exquisite control over the two pulses,” said Zhirong Huang, associate professor of physics at SLAC who coordinated the R&D efforts.
Custom diagnostic tools were critical in confirming the pulses were synchronized, he said. The well-controlled separation in the energies of the paired pulses will also be vital for clearly distinguishing pairs of spots, called “doublets,” in the X-ray crystallography patterns that form the basis for determining the 3-D structure.
Agostino Marinelli, a SLAC accelerator physicist who was lead author of the Nature Communications paper, said the new capability is already in demand: “We have already had three experiments, with four more experiments scheduled.”
Researchers at SLAC, the Stanford University School of Medicine and DESY lab in Germany collaborated in the R&D effort, which was supported by the U.S. Department of Energy Office of Science.
SLAC is a multi-program laboratory exploring frontier questions in photon science, astrophysics, particle physics and accelerator research. Located in Menlo Park, Calif., SLAC is operated by Stanford University for the U.S. Department of Energy's Office of Science.
SLAC National Accelerator Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.
Media Contact
All latest news from the category: Physics and Astronomy
This area deals with the fundamental laws and building blocks of nature and how they interact, the properties and the behavior of matter, and research into space and time and their structures.
innovations-report provides in-depth reports and articles on subjects such as astrophysics, laser technologies, nuclear, quantum, particle and solid-state physics, nanotechnologies, planetary research and findings (Mars, Venus) and developments related to the Hubble Telescope.
Newest articles

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…

Innovative microscopy demystifies metabolism of Alzheimer’s
Researchers at UC San Diego have deployed state-of-the art imaging techniques to discover the metabolism driving Alzheimer’s disease; results suggest new treatment strategies. Alzheimer’s disease causes significant problems with memory,…

A cause of immunodeficiency identified
After stroke and heart attack: Every year, between 250,000 and 300,000 people in Germany suffer from a stroke or heart attack. These patients suffer immune disturbances and are very frequently…





















