Aluminum alloy overcomes obstacles on the path to making hydrogen a practical fuel source

Now, a team of researchers from the University of Texas at Dallas and Washington State University in Pullman, Wash., has made the counterintuitive discovery that aluminum, with a minor modification, is able to both break down and capture individual hydrogen atoms, potentially leading to a robust and affordable fuel storage system.
In nature, when two atoms of hydrogen meet they combine to form a very stable molecule (H2). Molecular hydrogen, however, has to be stored under great pressure and at very low temperatures, which is impractical if you want to power a vehicle or provide electricity for a home. A better solution would be to find a material that, at easily maintained temperatures and pressures, could efficiently store individual hydrogen atoms and release them on demand.
The first step in this process – hydrogen activation, breaking the chemical bonds that hold two hydrogen atoms together – is typically done by exposing molecular hydrogen to a catalyst. The best catalytic materials currently available are made of so-called “noble metals” (e.g. palladium and platinum). These elements efficiently enable hydrogen activation, but their scarcity makes them prohibitively expensive for widespread use.
In the quest to find an equally efficient yet less-expensive alternative, lead researcher Yves J. Chabal of the University of Texas at Dallas and Santanu Chaudhuri at Washington State University have identified a potential new hydrogen activation method that has the additional advantage of being an effective hydrogen-storage medium. Their proposed system relies on aluminum, a plentiful but inert metal that under normal conditions doesn't react with molecular hydrogen.
The key to unlocking aluminum's potential, the researchers surmised, is to impregnate its surface with some other metal that would facilitate the catalytic reaction. In this case, the researchers tested titanium, which is much more plentiful than noble metals and is used only sparingly in creating the titanium-doped aluminum surface.
Under very controlled temperatures and pressures, the researchers studied the aluminum surface, particularly in the vicinity of the titanium atoms, for telltale signs that catalytic reactions were taking place. The “smoking gun” was found in the spectroscopic signature of carbon monoxide (CO), which was added to the system to help identify areas of hydrogen activity. If atomic hydrogen were present, then the wavelength of light absorbed by the carbon monoxide bound to the catalytic metal center would become shorter, signaling that the catalyst was working.
“We've combined a novel infrared reflection absorption-based surface analysis method and first principles-based predictive modeling of catalytic efficiencies and spectral response, in which a carbon monoxide molecule is used as a probe to identify hydrogen activation on single-crystal aluminum surfaces containing catalytic dopants,” says Chaudhuri.
Their studies revealed that in areas doped with titanium, the infrared signature of the CO shifted to shorter wavelengths even at very low temperatures. This “blue shift” was an indication that atomic hydrogen was being produced around some of the catalytic centers on an aluminum surface.
As part of a hydrogen storage system, an aluminum-supported catalyst has other advantages over more expensive metals. If technical advances like this can provide a pathway for aluminum to combine with hydrogen to form aluminum hydride (a stable solid with a composition ratio of a single aluminum atom to three hydrogen atoms) and store hydrogen as a high-density solid-state material, a critical step in developing a practical fuel system can be achieved.
The titanium further advances the process by helping the hydrogen bind to the aluminum to form aluminum hydride. If used as a fuel-storage device, the aluminum hydride could be made to release its store of hydrogen by simply raising its temperature.
“Although titanium may not be the best catalytic center for fully reversible aluminum hydride formation, the results prove for the first time that titanium-doped aluminum can activate hydrogen in ways that are comparable to expensive and less-abundant catalyst metals such as palladium and other near-surface alloys consisting of similar noble metals and their bimetallic analogs,” Chaudhuri explains.
Irinder Chopra, the lead student in this project, will present this research at AVS' 58th International Symposium & Exhibition, held Oct. 30 – Nov. 4, 2011, in Nashville, Tenn. A paper based on this research – “Turning Aluminum into a noble-metal like catalyst for low-temperature molecular hydrogen activation” –was published online in the journal Nature Materials on September 25. Support for this research came from the Department of Energy – Office of Basic Energy Sciences.
The AVS 58th International Symposium & Exhibition will be held Oct. 30 – Nov. 4 at the Nashville Convention Center.
Presentation SS1-TuM-4, “Turning Aluminum into a Noble-metal like Catalyst for Low Temperature Molecular Hydrogen Activation,” is at 9 a.m. on Tuesday, Nov. 1.
USEFUL LINKS:
Main meeting website: http://www2.avs.org/symposium/AVS58/pages/greetings.html
Technical Program: http://www2.avs.org/symposium
Media Contact
More Information:
http://www.aip.orgAll latest news from the category: Physics and Astronomy
This area deals with the fundamental laws and building blocks of nature and how they interact, the properties and the behavior of matter, and research into space and time and their structures.
innovations-report provides in-depth reports and articles on subjects such as astrophysics, laser technologies, nuclear, quantum, particle and solid-state physics, nanotechnologies, planetary research and findings (Mars, Venus) and developments related to the Hubble Telescope.
Newest articles
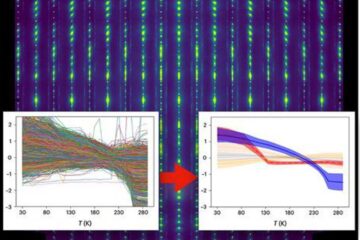
Machine learning algorithm reveals long-theorized glass phase in crystal
Scientists have found evidence of an elusive, glassy phase of matter that emerges when a crystal’s perfect internal pattern is disrupted. X-ray technology and machine learning converge to shed light…
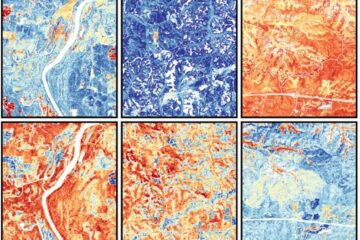
Mapping plant functional diversity from space
HKU ecologists revolutionize ecosystem monitoring with novel field-satellite integration. An international team of researchers, led by Professor Jin WU from the School of Biological Sciences at The University of Hong…

Inverters with constant full load capability
…enable an increase in the performance of electric drives. Overheating components significantly limit the performance of drivetrains in electric vehicles. Inverters in particular are subject to a high thermal load,…





















