Fast-tracking T cell therapies with immune-mimicking biomaterials
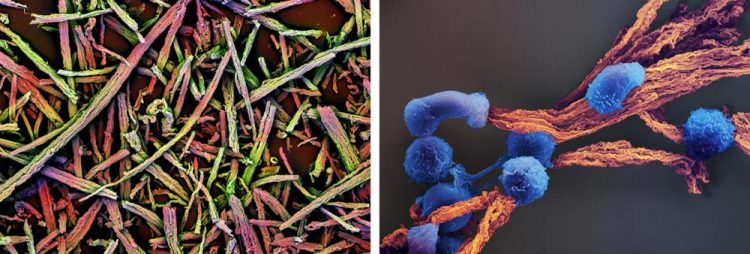
The left image shows a scanning electron micrograph (SEM) of a basic scaffold made of many tiny mesoporous silica rods (MSRs) before they are coated with a thin supported lipid bilayer (SLB) with the incorporated T cell-stimulating cues. In the right SEM image, T cells (in blue) bind to a section of a completed antigen-presenting cell-mimetic scaffold (in brown), where they are instructed to multiply and are kept alive for future use in T cell therapies. Credit: Wyss Institute at Harvard University.
Immunologists and oncologists are harnessing the body's immune system to fight cancers and other diseases with adoptive cell transfer techniques. In a normal immune response, a type of white blood cell known as T cells are instructed by another kind of immune cell called an antigen-presenting cell (APC) to expand their numbers and stay alive. Adoptive cell transfer procedures are mimicking exactly this process in a culture dish by taking T cells from patients, multiplying them, sometimes genetically modifying them, and then returning them to patients so that they can, for example, locate and kill cancer cells. However, these procedures often take weeks to produce batches of therapeutic T cells that are large and reactive enough to be able to eliminate their target cells.
A team led by David Mooney at Harvard's Wyss Institute for Biologically Inspired Engineering and John A. Paulson School of Engineering and Applied Sciences (SEAS) is now reporting in Nature Biotechnology an alternative material-based T-cell-expansion method that could help surmount these obstacles. With an APC-mimetic biomaterial scaffold, the researchers achieved greater expansion of primary mouse and human T cells than with existing methods; and they demonstrated the approach's potential in a mouse lymphoma model treated with chimeric antigen receptor-expressing T cells (CAR-T cells) that are engineered to home in on and destroy lymphoma cells.
“Our approach closely mimics how APCs present their stimulating cues to primary T cells on their outer membrane and how they release soluble factors that enhance the survival of the T cells. As a result, we achieve much faster and greater expansion. By varying the compositions of lipids, cues, and diffusible factors in the scaffolds, we engineered a very versatile and flexible platform that can be used to amplify specific T cell populations from blood samples, and that could be deployed in existing therapies such as CAR-T cell therapies,” said Mooney, Ph.D., a Core Faculty member at the Wyss Institute and leader of its Immunomaterials Platform. Mooney is also the Robert P. Pinkas Family Professor of Bioengineering at SEAS.
To engineer an APC-mimetic scaffold, the team first loaded tiny mesoporous silica rods (MSRs) with Interleukin 2 (IL-2) ¬– an APC-produced factor that prolongs the survival of associated T cells. The MSRs were then coated with lipids that formed a thin supported lipid bilayer (SLB), which resembles the outer membrane of APCs and that the researchers then functionalized with a pair of T cell-stimulating antibodies that remain mobile in the lipid layer and can bind to receptor/co-receptor molecules on the surface of T cells. In culture medium, 3D scaffolds spontaneously formed through the settling and random stacking of the rods, forming pores big enough to allow the entry, movement, and accumulation of T cells, thereby signaling them to multiply.
In a series of side-by-side comparisons, Mooney's team demonstrated that APC-mimetic scaffolds performed better than methods involving commercially available expansion beads (Dynabeads), which are currently used in clinical adoptive cell transfer approaches. “In a single dose, APC-mimetic scaffolds led to two- to ten-fold greater expansion of primary mouse and human T cells than Dynabeads. As another advantage, APC-mimetic scaffolds enabled us to tune the ratios of subpopulations of T cells with different roles in the desired immune responses, which in the future might increase their functionality,” said David Zhang, the study's second author and a Graduate Student working with Mooney.
Building on these findings, the researchers demonstrated the utility of their T cell expansion platform in a therapeutic model. “Prompted by recent breakthroughs in CAR-T cell therapies, we showed that a specific CAR-T cell product expanded with an APC-mimetic scaffold could facilitate treatment of a mouse model of a human lymphoma cancer,” said first author Alexander Cheung, Ph.D., who started the project in Mooney's team and now is a scientist at UNUM Therapeutics in Cambridge, Massachusetts. An APC-mimetic scaffold that was engineered to activate a specific type of CAR-T cell was able to generate higher numbers of the modified T cells over longer periods of culture than analogously designed expansion beads, and the resulting cells were similarly effective in killing the lymphoma cells in the mice.
After successfully using the material to expand all T cells present in a sample, the team demonstrated that APC-mimetic scaffolds could also be used to expand antigen-specific T cell clones from a more complex mixture of cells. Such T cell clones are constantly developed by the immune system to recognize small specific peptides contained in foreign proteins. To this aim, the researchers incorporated molecules into the scaffolds that are known as the major histocompatibility complex (MHC) and that presented small peptides derived from viral proteins to T cells.
“Based also on studies in which we showed that APC-mimetic scaffolds also have superior potential to specifically enrich and expand rare T cell sub-populations from blood, we strongly believe that we created an effective platform technology that could facilitate more effective precision immunotherapies,” said Cheung.
“The bioinspired T cell-activating scaffolds developed by the Wyss Institute's Immunomaterials Platform could accelerate the success of many immunotherapeutic approaches in the clinic, with life-saving impact on a broad range of patients, in addition to advancing personalized medicine,” said Wyss Institute Founding Director Donald Ingber, M.D., Ph.D., who is also the Judah Folkman Professor of Vascular Biology at HMS and the Vascular Biology Program at Boston Children's Hospital, as well as Professor of Bioengineering at SEAS.
###
In addition, Sandeep Koshy, Ph.D., who worked as a Graduate Student on Mooney's team and now is an Immuno-oncology Researcher at the Novartis Institutes for BioMedical Research in Cambridge, Mass., is an author on the study. The work was supported by the Wyss Institute for Biologically Inspired Engineering at Harvard University, the National Institutes of Health, and the National Science Foundation.
MULTIMEDIA AVAILABLE
PRESS CONTACT
Wyss Institute for Biologically Inspired Engineering at Harvard University Benjamin Boettner
617-432-8232
benjamin.boettner@wyss.harvard.edu
MULTIMEDIA CONTACT
Wyss Institute for Biologically Inspired Engineering at Harvard University Seth Kroll
617-432-7758
seth.kroll@wyss.harvard.edu
The Wyss Institute for Biologically Inspired Engineering at Harvard University uses Nature's design principles to develop bioinspired materials and devices that will transform medicine and create a more sustainable world. Wyss researchers are developing innovative new engineering solutions for healthcare, energy, architecture, robotics, and manufacturing that are translated into commercial products and therapies through collaborations with clinical investigators, corporate alliances, and formation of new startups. The Wyss Institute creates transformative technological breakthroughs by engaging in high risk research, and crosses disciplinary and institutional barriers, working as an alliance that includes Harvard's Schools of Medicine, Engineering, Arts & Sciences and Design, and in partnership with Beth Israel Deaconess Medical Center, Brigham and Women's Hospital, Boston Children's Hospital, Dana-Farber Cancer Institute, Massachusetts General Hospital, the University of Massachusetts Medical School, Spaulding Rehabilitation Hospital, Boston University, Tufts University, Charité – Universitätsmedizin Berlin, University of Zurich and Massachusetts Institute of Technology.
The Harvard John A. Paulson School of Engineering and Applied Sciences serves as the connector and integrator of Harvard's teaching and research efforts in engineering, applied sciences, and technology. Through collaboration with researchers from all parts of Harvard, other universities, and corporate and foundational partners, we bring discovery and innovation directly to bear on improving human life and society.
Media Contact
All latest news from the category: Health and Medicine
This subject area encompasses research and studies in the field of human medicine.
Among the wide-ranging list of topics covered here are anesthesiology, anatomy, surgery, human genetics, hygiene and environmental medicine, internal medicine, neurology, pharmacology, physiology, urology and dental medicine.
Newest articles

Silicon Carbide Innovation Alliance to drive industrial-scale semiconductor work
Known for its ability to withstand extreme environments and high voltages, silicon carbide (SiC) is a semiconducting material made up of silicon and carbon atoms arranged into crystals that is…

New SPECT/CT technique shows impressive biomarker identification
…offers increased access for prostate cancer patients. A novel SPECT/CT acquisition method can accurately detect radiopharmaceutical biodistribution in a convenient manner for prostate cancer patients, opening the door for more…

How 3D printers can give robots a soft touch
Soft skin coverings and touch sensors have emerged as a promising feature for robots that are both safer and more intuitive for human interaction, but they are expensive and difficult…





















