Zooming in on the Weapons of Salmonella
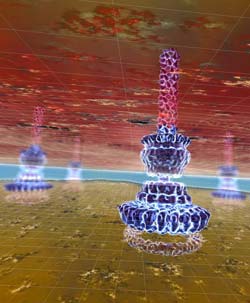
Structure of the needle-complex of Salmonella, embedded in a cellular context (artist’s interpretation based on original data). IMP-IMBA<br>
A group of Vienna-based scientists headed by Thomas Marlovits employed recently developed methods of cryo-electron microscopy and have been able to clarify the structure of this infection apparatus on the near-atomic scale. The exact knowledge of the needles’ building plan may help to develop substances that interfere with its function and thus prevent infection.
“Open Sesame” for bacteria
Some of the most dreaded diseases in the world such as plague, typhoid and cholera are caused by bacteria that have one thing in common: they possess an infection apparatus which is a nearly unbeatable weapon. When attacking a cell of the body, they develop numerous hollow-needle-shaped structures that project from the bacterial surface. Through these needles, the bacteria inject signal substances into the host cells, which re-program the latter and thereby overcome their defense. From this time on it's easy game for the pathogens; they can invade the cells unimpeded and in large numbers.
The biochemist and biophysicist Thomas Marlovits, a group leader at the Vienna Institutes IMP (Research Institute of Molecular Pathology) and IMBA (Institute of Molecular Biotechnology) has been occupied for several years with the infection complex of salmonellae. As early as in 2006 Thomas Marlovits showed how the needle complex of Salmonella typhimurium develops (Nature 441, 637-640). Together with his doctoral student Oliver Schraidt he has now been able to demonstrate the three-dimensional structure of this complex in extremely high resolution. The team was able to show details with dimensions of just 5 to 6 angstroems, which are nearly atomic orders of magnitude. Their work will be presented in the forthcoming issue of the journal Science.
Looks do kill!
Never before has the infection tool of salmonellae been presented in such precision. This was achieved by the combined use of high-resolution cryo-electron microscopy and specially developed imaging software. “Austria’s coolest microscope” makes it possible to shock-freeze biological samples at minus 196 degrees centigrade and view them in almost unchanged condition. However, when “zooming in” on their object, scientists are confronted with a treacherous problem: the high-energy electron beam falls at such high concentrations on the sample that the latter is destroyed after the very first image.
The Viennese scientists have resolved the problem by developing new image-processing algorithms and with sheer numbers of images. They analyzed about 37,000 images of isolated needle complexes. Similar images were grouped and computed jointly. By doing so they were able to generate a single sharp image from numerous blurred ones. This enormous computing power was created by a cluster of about 500 interconnected computers.
Microscopy without the human interference factor
The microscope works in semi-automated fashion at night to obtain the large number of images. This is very advantageous because human beings merely interfere with the job. They breathe, speak, move, and thus unsettle the sensitive microscope. Even a moving elevator may irritate the electron beam.
The cryo-electron microscope at IMP-IMBA is the only one of its kind in Austria. The immense technical effort associated with its operation pays off, as far as the scientists are concerned. Advancing into the subnanometer range created a further means of expanding their knowledge. They were able to “adjust” existing data (obtained from crystallography) to the needle structure and thus complement the three-dimensional image in a perfect manner. The use of this hybrid method enabled the scientists to elucidate the complete construction plan of the infection apparatus.
Thomas Marlovits regards this technology as an innovation boost: “Using the methods we developed for our work, we were able to establish “imaging” standards at a very high level. We can explore its absolute limits with the aid of the fantastic infrastructure we have here at Campus Vienna Biocenter.”
This knowledge not only advances basic research. “Using our data, we may well be able to find a compound that interferes with the needle complex and disturb its function,” says Marlovits. “We would then have a very effective medication – one that combats not only salmonellae but also other pathogens that employ this system, such as pathogens that cause cholera, plague or typhoid.”
The biochemist Thomas Marlovits was born in Rechnitz, Austria. He is a joint group leader of the two institutes IMP and IMBA since 2005. Previously, he spent five years as a post-doctoral student at the University of Yale. Thomas Marlovits has been occupied with the structure and function of molecular machines. He started to investigate the infection apparatus of salmonellae at Yale and continued this work at IMP-IMBA.
Thomas Marlovits' research work is supported within the scope of “Vienna Spots of Excellence” as part of the “Center of Molecular and Cellular Nanostructure Vienna (CMCN)”, headed by Thomas Marlovits. This initiative of the City of Vienna supports research projects which involve both enterprises and scientific partners.
Original paper: “Three-Dimensional Model of Salmonella’s Needle Complex at Subnanometer Resolution”. Oliver Schraidt & Thomas C. Marlovits, Science 331, pp. 1192-1195, March 4, 2011.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















