Wood instead of petroleum: New approach to producing chemical substances from renewable resources
Molecules from wood: Production of active substances from wood-based starting materials Ill./©: Jason W. Runyon
Petroleum might well be replaced by wood soon when it comes to manufacturing chemical substances. Research has now made significant progress towards using sustainable biomass, like wood, as an alternative raw material for chemical production.
Scientists at Johannes Gutenberg University Mainz (JGU) in Germany and at the University of Alabama in Tuscaloosa in the USA recently managed to synthesize two complex chemical substances from wood-based starting materials.
The process can be as cost-effective as the conventional petroleum product-based process and is less damaging to the environment. “Our aim is to manufacture everyday products from renewable resources without an impact on the environment while at the same time ensuring that the process is economically competitive,” explained Professor Till Opatz of Mainz University. The results of their research have been published in the prominent journal Angewandte Chemie.
The German research team led by Professor Till Opatz at JGU's Institute of Organic Chemistry participates in the interdisciplinary research consortium Chemical BioMedicine (ChemBioMed) funded by the Carl Zeiss Foundation and works on the synthesis of substances that can inhibit tumor cell growth.
The US research group under Professor Anthony J. Arduengo III is particularly interested in developing industrially applicable methods for using materials derived from wood biomass for the sustainable manufacture of a broad array of basic chemicals such as, for example, substances used to produce automotive coatings, plastics, adhesives, and other commodity materials.
At a conference in Goslar in Germany about two years ago, the two researchers realized that their experience and expertise complemented each other perfectly for addressing issues surrounding sustainability of a modern chemical industry. Since then, a vigorous exchange of researchers and students between Mainz and Tuscaloosa has fueled the collaboration.
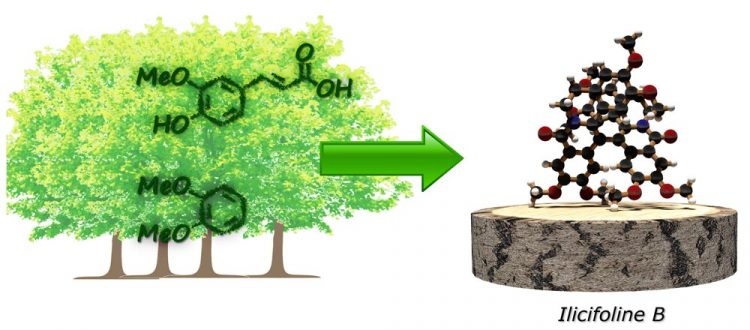
The two teams have now been able to demonstrate wood-based, or xylochemical, syntheses of substances for which petroleum products are usually employed as starting materials. This new work shows that the relevant carbon skeletons can be created solely from wood-based starting materials. In the case of one target compound, the natural product ilicifoline B, no comparison with a petrochemical route was possible as this substance had never before been synthesized in a laboratory. But when it came to derivatives of the natural painkiller morphine, the new xylochemical synthesis turned out to be significantly more efficient than any previously known route based on petrochemistry.
“This shows that the implementation of a wood-based chemical economy is not necessarily associated with decreased cost-efficiency,” added Daniel Stubba, JGU first author of the publication. “Xylochemistry could represent an important alternative to the climate-damaging use of the earth's finite resources of natural oil and gas in the production of chemicals.”
Further related research is ongoing in the two laboratories and additional international collaborators have been recruited to address a broader range of connected topics. For this latter purpose, an international research consortium called StanCE (Sustainable Technology for a new Chemical Economy) has been established. It brings together researchers from the USA, Germany, Japan, and Canada who are collaborating on the development of an alternative, sustainable chemical infrastructure that does not consume finite resources and avoids ecological imbalances while remaining cost-efficient.
Wood contains a variety of potential starting materials that, because of their chemical structure, are better suited than petroleum products for many applications. It is often necessary to subject the latter to extensive transformation processes before they acquire comparable functionality.
“Wood is the ideal raw material because it is renewable and an easily accessible resource at the same time. Its composition is like a box of varied building blocks from which products for today's modern world can be manufactured,” said Opatz, adding that Alabama and Germany, like Canada, have extensive available wood resources.
Illustration:
http://www.uni-mainz.de/bilder_presse/09_orgchemie_xylochemie.jpg
Molecules from wood: Production of active substances from wood-based starting materials
Ill./©: Jason W. Runyon
Publication:
Daniel Stubba et al.
Xylochemistry – Making Natural Products Entirely from Wood
Angewandte Chemie, 21 October 2015
DOI: 10.1002/anie.201508500
Further information:
Professor Dr. Till Opatz
Institute of Organic Chemistry
Johannes Gutenberg University Mainz (JGU)
D 55099 Mainz, GERMANY
phone +49 6131 39-22272 or 39-24443
fax +49 6131 39-22338
e-mail: opatz@uni-mainz.de
http://www.chemie.uni-mainz.de/OC/AK-Opatz/index_ENG.php
Related links:
http://onlinelibrary.wiley.com/doi/10.1002/anie.201508500/abstract
http://www.wiley-vch.de/util/hottopics/suschem/
http://www.uni-mainz.de/presse/19700_ENG_HTML.php – press release
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Bringing bio-inspired robots to life
Nebraska researcher Eric Markvicka gets NSF CAREER Award to pursue manufacture of novel materials for soft robotics and stretchable electronics. Engineers are increasingly eager to develop robots that mimic the…

Bella moths use poison to attract mates
Scientists are closer to finding out how. Pyrrolizidine alkaloids are as bitter and toxic as they are hard to pronounce. They’re produced by several different types of plants and are…

AI tool creates ‘synthetic’ images of cells
…for enhanced microscopy analysis. Observing individual cells through microscopes can reveal a range of important cell biological phenomena that frequently play a role in human diseases, but the process of…





















