Water is not the same as water
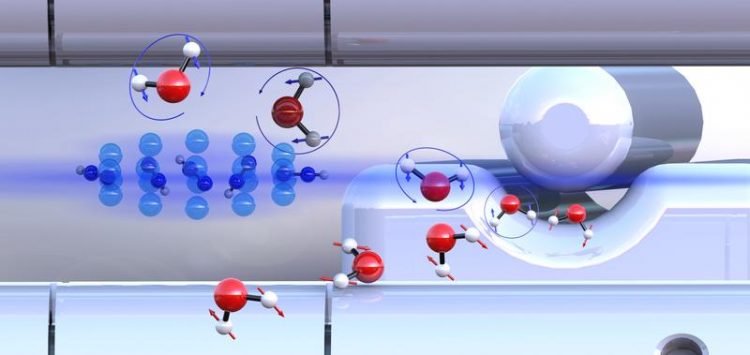
Pre-sorted ortho-water and para-water molecules with differently oriented nuclear spins (blue or red arrows) react with diazenylium ions (center left) at different speeds. University of Basel
From a chemical perspective, water is a molecule in which a single oxygen atom is linked to two hydrogen atoms. It is less well known that water exists in two different forms (isomers) at the molecular level. The difference lies in the relative orientation of the nuclear spins of the two hydrogen atoms. Depending on whether the spins are aligned in the same or opposite direction, one refers to ortho- or para-water.
Experiments with sorted water molecules
The research group headed by Professor Stefan Willitsch from the University of Basel’s Department of Chemistry has investigated how the two forms of water differ in terms of their chemical reactivity – their ability to undergo a chemical reaction. Both isomers have almost identical physical properties which makes their separation particularly challenging.
This separation was made possible by a method based on electric fields developed by Professor Jochen Küpper from the Hamburg Center for Free-Electron Laser Science. Using this approach, the researchers were able to initiate controlled reactions between the “pre-sorted” water isomers and ultracold diazenylium ions (“protonated nitrogen”) held in a trap. During this process, a diazenylium ion transfers its proton to a water molecule. This reaction is also observed in the chemistry of interstellar space.
Increased reactivity
It was demonstrated that para-water reacts about 25% faster than ortho-water. This effect can be explained in terms of the nuclear spin also influencing the rotation of the water molecules. As a result, different attractive forces act between the reaction partners. Para-water is able to attract its reaction partner more strongly than the ortho-form, which leads to an increased chemical reactivity. Computer simulations confirmed these experimental findings.
In their experiments, the researchers worked with molecules at very low temperatures close to the absolute zero point (about –273°C). These are ideal conditions to precisely prepare individual quantum states and define the energy content of the molecules, and to cause a controlled reaction between them. Willitsch explains the experimental approach: “The better one can control the states of the molecules involved in a chemical reaction, the better the underlying mechanisms and dynamics of a reaction can be investigated and understood.”
Original Source
Ardita Kilaj, Hong Gao, Daniel Rösch, Uxia Rivero, Jochen Küpper, Stefan Willitsch
Observation of different reactivities of para- and ortho-water towards trapped diazenylium ions
Nature Communications (2018), doi: 10.1038/s41467-018-04483-3
Further Information
Prof. Dr. Stefan Willitsch, Universität Basel, Departement Chemie, Tel. +41 61 207 38 30, E-Mail: stefan.willitsch@unibas.ch
Prof. Dr. Jochen Küpper, Center for Free-Electron Laser Science, Hamburg, Tel. +49 40 8998-6330 , E-Mail: jochen.kuepper@cfel.de
Media Contact
More Information:
http://www.unibas.chAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















