Understanding PP1, the ubiquitous enzyme
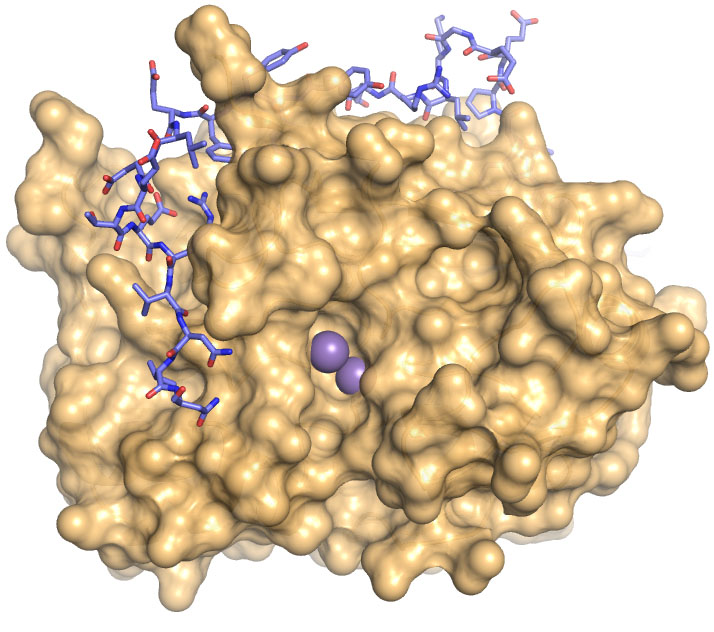
The Enzyme PP1, the tan mass above, is everywhere in the body and has a role in nearly every biological process. That role is shaped more than 200 regulatory Proteins that bind to PP1, including one called PNUTS. blue and pink above. Credit: Page lab/Brown University
In the Proceedings of the National Academy of Sciences, a team of scientists at Brown University reports a major step forward in determining the specific behavior of the ubiquitous enzyme PP1 implicated in a wide range of diseases including cancer.
PP1, whose role is to enable the passage of molecular messages among cells, is found pretty much everywhere in the body. Its wide range of responsibilities means it is essential to many healthy functions and, when things go wrong, to diseases. But its very versatility has prevented it from being a target for drug development, said Rebecca Page, associate professor of biology at Brown and the paper’s corresponding author.
“The amazing thing about PP1 is that no one has wanted to touch it for the most part as a drug target because PP1 is involved in nearly every biological process,” Page said. “It’s not like you could just target the PP1 active site for, let’s say, diabetes because then you are going to affect drug addiction, Alzheimer’s disease and all these other diseases at the same time.”
In other words, make a medicine to block PP1 in one bodily context and you’d ruin it in all other contexts. Structural biologists such as Page and Brown co-author Wolfgang Peti have therefore been eager to learn what makes PP1 behave in specific ways in specific situations.
The key is the way PP1 binds with more than 200 different regulatory proteins. Scientists know of these proteins and know the sequences of amino acids that compose them, but they don’t know their structure or how they actually guide PP1.
“The ability to predict how these PP1 interacting proteins bind PP1 from sequence alone is still missing,” Page and her colleagues wrote in PNAS.
Now, through experiments in which her team including lead author Meng Choy combined NMR spectroscopy, X-ray crystallography and techniques in biochemistry, she has learned how PP1 binds to a targeting protein called PNUTS, forming “binding motifs.” That knowledge, combined with what she learned in earlier studies about two other targeting proteins — NIPP1 and spinophilin — has allowed her team to predict how PP1 binds with 43 of the 200 regulatory proteins that give it specific behavior.
“What this work in conjunction with two of our previous structures allowed us to do was to define two entirely new motifs,” she said. “From that, comparing the sequences with the known proteins that interact with PP1 whose structures we don’t have, we were able to predict that 20 percent of them likely interact in a way that is similar to these three proteins.”
So by resolving the structure of just three proteins with PP1, Page now has the means to understand the binding of many proteins without having to resolve their structure. Instead she need only know the few motifs and the proteins’ sequences.
As for PP1’s interactions with the other 80 percent or so of regulatory proteins, those remain a mystery. But Page said the success her team has had in the lab working with PP1 and resolving key motifs makes her optimistic that those interactions can be solved, too.
In addition to Page, Choy, and Peti, the paper’s other authors are Martina Hieke, Ganesan Senthil Kumar, Greyson Lewis, Kristofer Gonzalez-DeWhitt, Rene Kessler, Benjamin Stein, and Manuel Hessenberger of Brown and Angus Nairn of Yale.
The National Institute of General Medical Sciences (grant R01GM098482) supported the research.
Media Contact
More Information:
http://news.brown.edu/pressreleases/2014/03/pp1All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

“Nanostitches” enable lighter and tougher composite materials
In research that may lead to next-generation airplanes and spacecraft, MIT engineers used carbon nanotubes to prevent cracking in multilayered composites. To save on fuel and reduce aircraft emissions, engineers…

Trash to treasure
Researchers turn metal waste into catalyst for hydrogen. Scientists have found a way to transform metal waste into a highly efficient catalyst to make hydrogen from water, a discovery that…

Real-time detection of infectious disease viruses
… by searching for molecular fingerprinting. A research team consisting of Professor Kyoung-Duck Park and Taeyoung Moon and Huitae Joo, PhD candidates, from the Department of Physics at Pohang University…





















