The nucleolus – a known organelle with new tasks
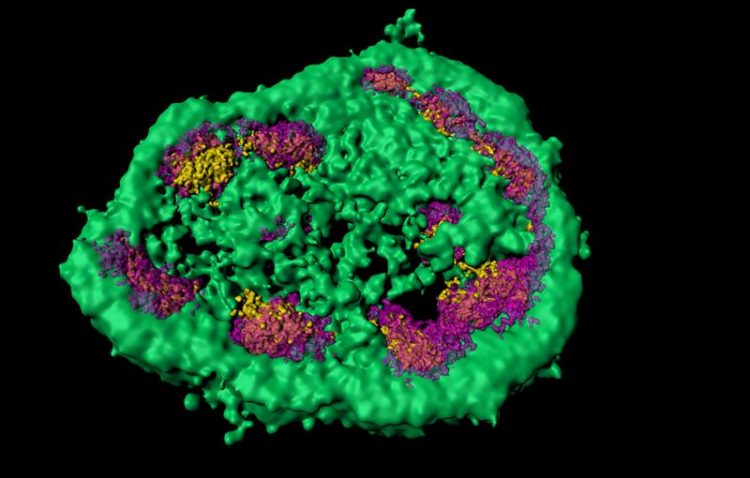
Super-resolution microscopy shows that the nucleolus consists of different membrane-less zones (here marked green, magenta and yellow). Misfolded proteins are temporarily stored in the green area. © MPI of Biochemistry
One would like to believe that the basic cellular processes have already been deciphered and that research can now focus on the details. But even today, new fundamental principles are being discovered through the combination of modern methods.
The nucleolus is a nuclear structure that was first described in the 1830s. In the 1960s it was recognized that ribosomes, the protein factories, are produced in this organelle.
Researchers have known for some time that protein folding helpers, so-called chaperones, move into the nucleolus under certain circumstances.
It has been suggested that this relocation is related to protein production. Now researchers from the Max Planck Institute (MPI) of Biochemistry have shown that the chaperones that move into the nucleolus are bound to stress-sensitive proteins.
As pioneer of chaperone research, F.-Ulrich Hartl and his team have discovered that chaperones are crucial for the correct folding of proteins and play a central role in the quality control of proteins. If protein folding does not work correctly, misfolded proteins can accumulate and clump together. The resulting protein aggregates can often be observed in neurodegenerative diseases such as Alzheimer's, Parkinson's or Huntington's disease.
Mark Hipp, corresponding author of the study and member of F.-Ulrich Hartl's department, comments: “We have been using luciferase as a model protein for many years in order to investigate the mechanisms of protein folding”.
Bound to a fluorescent protein, the scientists can see under the microscope whether the protein is correctly folded or if it is misfolded and aggregates. “We were able to show that stressing cells by heating them to 43°C, results in the transport of the misfolded luciferase protein together with the chaperones into the nucleolus.”
The researchers cooperated with the groups of Ralf Jungmann, developer of high-resolution fluorescence methods, and Jürgen Cox, developer of bioinformatic analysis methods, both also located at the MPI of Biochemistry, in order to elucidate the mechanistic details of this process. Together they were able to show that the misfolded luciferase protein behaved differently within the nucleolus than in the rest of the cell.
“In the nucleolus, misfolded proteins were kept in a liquid-like state instead of aggregating,” explains Frédéric Frottin, first author of the study. This is possible due to the specific biophysical conditions of this organelle.
“Proteins that usually tend to aggregate are stored in a less dangerous form during the stress which protects cells from damage. Once the cell had time to recover, the proteins can be refolded and released from the nucleolus,” continues Frottin. Now, the cells have the capacity to activate further mechanisms that enable the protein to be repaired or degraded.
The researchers could also show that this protective mechanism fails if the cell stress lasts too long. “This is a new mechanism that maintains the integrity of the cell,” says Mark Hipp. Maintaining this integrity is ultimately essential to prevent aging and the development of disease.
Dr. Mark Hipp
Department of Cellular Biochemistry
Max Planck Institute of Biochemistry
Am Klopferspitz 18
82152 Martinsried/Germany
E-mail: hipp@biochem.mpg.de
https://www.biochem.mpg.de/en/rd/hartl/mark_hipp
Prof. Dr. F.-Ulrich Hartl
Department of Cellular Biochemistry
Max Planck Institute of Biochemistry
Am Klopferspitz 18
82152 Martinsried
Germany
E-Mail: uhartl@biochem.mpg.de
www.biochem.mpg.de/hartl
Prof. Dr. Ralf Jungmann
Molecular Imaging and Bionanotechnology
Max Planck Institute of Biochemistry
Am Klopferspitz 18
82152 Martinsried
Germany
E-Mail: jungmann@biochem.mpg.de
http://www.biochem.mpg.de/jungmann
F. Frottin, F. Schueder, S. Tiwary, R. Gupta, R. Körner, T. Schlichthaerle, J. Cox, R. Jungmann, F.U. Hartl, M.S. Hipp: The nucleolus functions as a phase-separated protein quality control compartment. Science. July 2019
DOI: https://doi.org/10.1126/science.aaw9157
Media Contact
More Information:
http://www.biochem.mpg.de/All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















