Supercapacitors turbocharged by laxatives
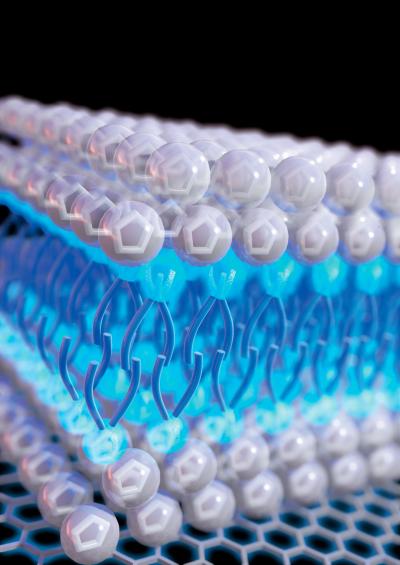
This is an illustration of detergent-like ionic liquids on an electrode surface. Credit: Xianwen Mao/Massachusetts Institute of Technology Usage Restrictions: Single use only - for use with the press release.
Their paper, published today in the journal Nature Materials, explains why these detergents, called ionic liquids, are better electrolytes than current materials and can improve supercapacitors.
Currently, aqueous and organic electrolytes are used, but more recently, researchers and manufacturers have been testing ionic liquids instead to boost performance.
Although ionic liquids are salts, at room temperature they are surprisingly not crystalline solids – as their name suggests they are in fact liquids.
This gives ionic liquids numerous advantages over conventional electrolytes because they are stable, non-flammable, and often much more environmentally friendly.
To explore the exciting potential offered by ionic liquids for emerging electrochemical technologies the authors designed a new set of highly efficient detergent-like ionic liquid electrolytes and explained how they work at electrode surfaces.
Understanding how they operate will help design even more efficient devices for storing electrical energy.
Professor Julian Eastoe, from the University of Bristol's School of Chemistry, is a co-author of the study. He said: “To make this discovery required a team of scientists with a very diverse skill set, spanning chemical synthesis, advanced structural, microscopy and electrical techniques as well as computational methods.
“This work demonstrates the power of scientific research 'without borders', the groups from different nations contributed their own expertise to make 'the whole greater than the sum of parts'.”
Co-author, Xianwen Mao, from the Massachusetts Institute of Technology (MIT), added: “We engineered a new class of ionic liquids that can store energy more efficiently.
“These detergent-like ionic liquids can self-assemble into sandwich-like bilayer structures on electrode surfaces. And that is very reason why they give better energy storage performance.”
Typically, for electrolytes in contact with a charged electrode, the distribution of ions is dominated by electrostatic Coulombic interactions.
However, this distribution can be controlled by making the ionic liquids soap-like, or amphiphilic, so that the molecules now have separate polar and non-polar domains, exactly like common detergents.
These soap-like electrolytes then spontaneously form bilayer structures on the electrode surfaces, leading to much improved energy storage capabilities. The researchers found that temperature and applied voltage also affect the energy storage performance.
This new class of electrolytes may be suitable for challenging operations, such as oil drilling and space exploration, but they may also pave the way to new and improved supercapacitors in hybrid cars.
These devices are essential components in modern hybrid cars and can outperform batteries in terms of higher power and better efficiency.
This is particularly the case during regenerative braking where mechanical work is turned into electrical energy, which can be stored quickly in supercapacitors ready to be released.
This reduces energy consumption and is much more environmentally friendly. More importantly, using the new electrolytes such as developed in this study, future supercapacitors may even be able to store more energy than batteries, potentially replacing batteries in applications such as electrical vehicles, personal electronics, and grid-level energy storage facilities.
Media Contact
More Information:
https://www.eurekalert.org/pub_releases/2019-08/uob-stb080919.phpAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Solving the riddle of the sphingolipids in coronary artery disease
Weill Cornell Medicine investigators have uncovered a way to unleash in blood vessels the protective effects of a type of fat-related molecule known as a sphingolipid, suggesting a promising new…

Rocks with the oldest evidence yet of Earth’s magnetic field
The 3.7 billion-year-old rocks may extend the magnetic field’s age by 200 million years. Geologists at MIT and Oxford University have uncovered ancient rocks in Greenland that bear the oldest…

Decisive breakthrough for battery production
Storing and utilising energy with innovative sulphur-based cathodes. HU research team develops foundations for sustainable battery technology Electric vehicles and portable electronic devices such as laptops and mobile phones are…





















