Structure of an iron-transport protein revealed
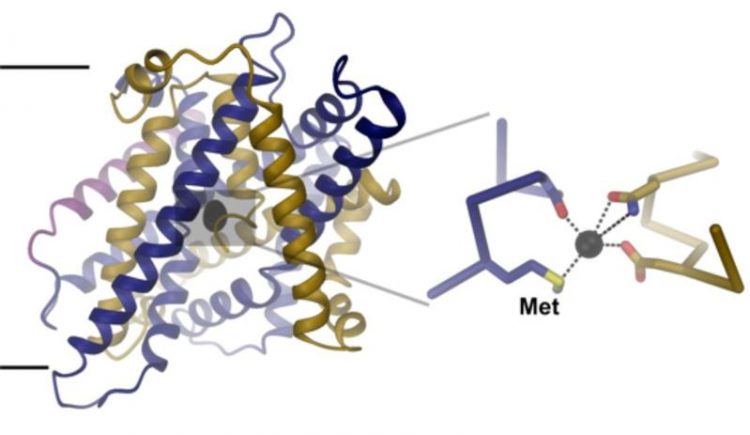
Structure of the iron transport-protein. A zoom into the iron binding-site (right) shows the interaction of the bound ion with conserved amino-acids. UZH
Iron is the most abundant trace element in humans. As a cofactor of certain proteins, it plays an essential role in oxygen transport and metabolism. Due to the major im-portance of iron in a wide variety of cellular processes, and the harm caused by its uncontrolled accumulation in the body, its uptake and storage is strictly regulated.
In mammals, iron is imported into cells by the membrane transport protein DMT1. Mu-tations of DMT1, which affect its transport properties, lead to iron-related metabolic disorders such as anemia and the iron storage disease hemochromatosis.
Ines Ehrnstorfer, a PhD student in the group of Professor Raimund Dutzler at the Department of Biochemistry of the University of Zurich, and her colleagues, have determined the first structure of an iron transport protein. Their work was published in the scientific journal Nature Structural and Molecular Biology. Based on these re-sults the researchers were able to explain why DMT1 binds the divalent metal ions iron and manganese (Fe2+ and Mn2+), but not calcium (Ca2+) – in spite of the latter being several orders of magnitude more abundant.
Moleclar basis for selective ion transport
To unravel the structural basis for this ion selectivity, Ines Ehrnstorfer has deter-mined the structure of a close bacterial homologue of DMT1 by X-ray crystallography. The transport protein contains an ion binding site located at the center of the mem-brane that is composed of conserved amino acids.
“One of these amino acids, a me-thionine, only interacts with transition-metal ions, but not with Ca2+”, explains Ehrn-storfer. The study also shows that mutations in the binding site weaken ion binding and transport in both the bacterial homologue and human DMT1.
“The results thus reveal how transition-metal ions such as iron are selectively trans-ported across the membrane, and they provide a basis for the development of spe-cific inhibitors of DMT1 for the treatment of iron storage diseases,” says the re-searcher.
The project was funded by the Swiss National Science Foundation through the National Center of Competence in Research (NCCR) TransCure.
Literature:
Ines A Ehrnstorfer, et al. Crystal structure of a SLC11 (NRAMP) transporter reveals the basis for transition-metal ion transport. Nature Structural and Molecular Biology, advanced online publication October 19 2014. Doi: 10.1038/nsmb.2904
Contacts:
Prof. Raimund Dutzler
Department of Biochemistry
University of Zurich
Tel.: +41 44 635 65 50
Email: dutzler@bioc.uzh.ch
Bettina Jakob
Media Relations
University of Zurich
Tel.: +41 44 634 44 39
Email: bettina.jakob@kommunikation.uzh.ch
http://www.mediadesk.uzh.ch/articles/2014/struktur-des-eisen-transportproteins-e…
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















