Speeding up the hydrogen production by the magic topological surface states
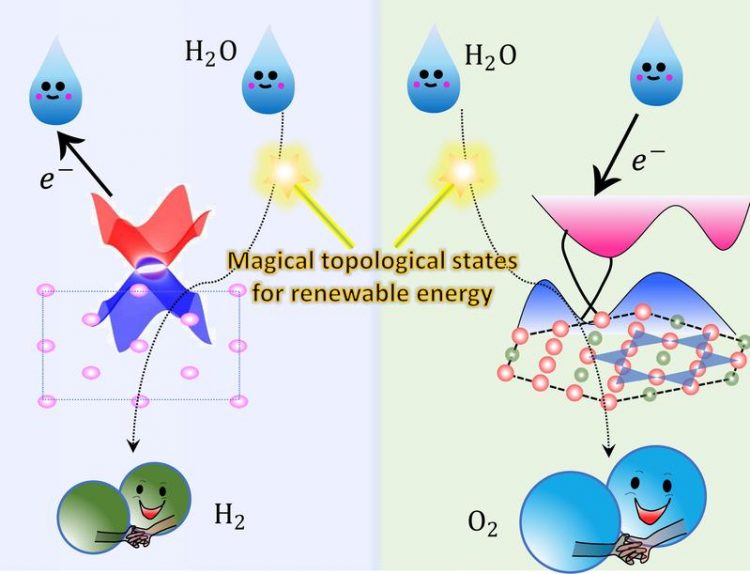
Topological non-trivial surface states can accept or donate electrons during the water electrolysis process. MPI CPfS
Water electrolysis could provide high-quality hydrogen gas that can be used in fuel cells directly. However, since noble metals, such as platinum and iridium, are currently needed to initiate such a reaction, the cost is very high.
“Obviously, catalysts that are low-cost with high-activity are needed to make hydrogen energy more competitive with traditional technologies,” says Guowei Li at the Max Planck Institute for Chemical Physics of Solids, who studied the surface reactions of several topological materials.
It was obviously a great challenge to find alternatives beyond noble metals.
“Topology may be the key to unlocking the barrier in the search for ideal catalysts,” says Prof. Claudia Felser, the director of Max Planck Institute for Chemical Physics of Solids. “We studied the surface properties of materials with topological order, from topological insulators to topological semimetals and metals, all these materials have non-trivial surface states that are protected by symmetries.”
“In other words, these surface states are very stable and robust against surface modifications such as impurity scattering and even oxidation: the question we’re asking is can we find such a perfect system that combines topological order, lost-cost, high efficiency, and high stability.”
The team from the Max Planck Institute Chemical Physics of Solids, Dresden together with colleagues from the TU Dresden and the Max Planck Institute for Microstructure Physics and Max-Planck-Institut für Kohlenforschung, Mülheim published a breakthrough result in Science Advances concerning a topological material, namely a magnetic Weyl-semimetal, that is a superior oxygen evolution reaction (OER) catalyst. The magnetic weyl semimetal that the team identified is Co3Sn2S2, a Kagome-lattice Shandite compound.[1]
High-quality bulk single crystals of Co3Sn2S2with sizes of up to centimeters can be exfoliated into thin-layers with defined crystal surfaces. The team showed that these surfaces act as superior catalysts for water splitting, even though the surface area is several orders of magnitudes smaller than today´s conventional nano-structured catalysts.
In collaboration with Yan Sun‘s theory group from the Max Planck Institute Chemical Physics of Solids, they found that there are cobalt-derived topological surface states just above the Fermi level.
In the water oxidation process, these surface states can accept electrons from the reaction intermediates, acting as an electron channel whose resistance is not affected by the harsh electrochemical environment.
Inspired by this strategy, the team then investigated the catalytic performance of a Dirac nodal arc semimetal PtSn4, a compound that has much lower percentage of expensive platinum.[2] Such crystals showed superior electrocatalytic stability for periods of time exceeding one month.
“The work serves as an interesting lens into the chemistry of these reaction processes and could be a pathway towards understanding the chemistry itself by clear knowledge of the topological nature of the semimetal catalyst,“ says one of the expert reviewers of the paper.
The research at the Max Planck Institute for Chemical Physics of Solids (MPI CPfS) in Dresden aims to discover and understand new materials with unusual properties.
In close cooperation, chemists and physicists (including chemists working on synthesis, experimentalists and theoreticians) use the most modern tools and methods to examine how the chemical composition and arrangement of atoms, as well as external forces, affect the magnetic, electronic and chemical properties of the compounds.
New quantum materials, physical phenomena and materials for energy conversion are the result of this interdisciplinary collaboration.
The MPI CPfS is part of the Max Planck Society and was founded in 1995 in Dresden. It consists of around 280 employees, of which about 180 are scientists, including 70 doctoral students.
Guowei Li
Claudia Felser
https://advances.sciencemag.org/content/5/8/eaaw9867
https://onlinelibrary.wiley.com/doi/full/10.1002/anie.201906109
http://www.cpfs.mpg.de
https://www.cpfs.mpg.de/3075686/20190816
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Machine learning algorithm reveals long-theorized glass phase in crystal
Scientists have found evidence of an elusive, glassy phase of matter that emerges when a crystal’s perfect internal pattern is disrupted. X-ray technology and machine learning converge to shed light…

Mapping plant functional diversity from space
HKU ecologists revolutionize ecosystem monitoring with novel field-satellite integration. An international team of researchers, led by Professor Jin WU from the School of Biological Sciences at The University of Hong…

Inverters with constant full load capability
…enable an increase in the performance of electric drives. Overheating components significantly limit the performance of drivetrains in electric vehicles. Inverters in particular are subject to a high thermal load,…





















