Research alliance launched, aimed at establishing bacteriophages as an approved drug
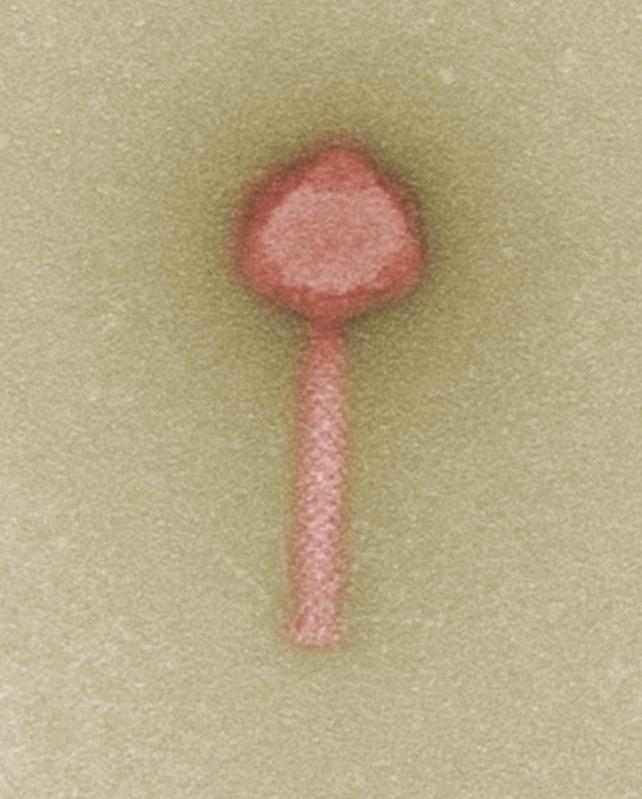
A candidate for future phage-based therapies: the bacteriophage that targets multiresistant clinical strains of Pseudomonas bacteria. © M. Rohde / HZI
The aim of the German research project “Phage4Cure” is to establish bacteriophages as an approved drug in the fight against infection. Bacteriophages are viruses that highly specifically recognize and bind to a certain type of bacteria, eventually causing their destruction. I
n eastern Europe in particular, phages have been successfully used for decades already as an alternative or complementary treatment to traditional antibiotic therapy. In the European Union, however, they have not yet been approved as drugs.
This is due, among other reasons, to missing quality standards for bacteriophage production, a sine qua non for drug approval by the authorities. Furthermore, systematic clinical trials first have to be performed to demonstrate the safety, tolerability, and efficacy of treatment with phages. This is exactly the aim the project partners are now going for.
The scientists will explore bacteriophages specifically targeting the bacterium Pseudomonas aeruginosa. This bacterium frequently becomes multiresistant and can cause, for example, lung infections. “Our goal in the medium term is to develop phages as a medicinal product that can be delivered via different routes of administration, to provide a novel and additional therapeutic option for different infectious diseases – in particular for those cases where antibiotics have reached their limits,” says Dr. Holger Ziehr, project coordinator and director of the Fraunhofer ITEM Division of Pharmaceutical Biotechnology.
In the project “Phage4Cure”, the four partners with their special know-how are each working on a different aspect of this topic. The Leibniz Institute DSMZ working group under Dr. Christine Rohde will identify bacteriophages targeting Pseudomonas aeruginosa, including genetic characterization.
“There are many different Pseudomonas aeruginosa strains, with only minor differences between them. The challenge is to identify phages with as broad a spectrum of hosts as possible,” the scientist explains. The phages will then be passed on to Fraunhofer ITEM for further high-quality purification and pharmacological production.
The Fraunhofer team under Dr. Holger Ziehr will develop a so-called platform manufacturing process for phage-based therapeutic agents. This means that the manufacturing process can subsequently be used for other phages as well. In addition, the Fraunhofer ITEM scientists will perform preclinical testing.
Further preclinical tests will be conducted at Charité – Universitätsmedizin Berlin by scientists from the team of Professor Martin Witzenrath, Deputy Director of the Medical Department, Division of Infectiology and Pneumonology. Professor Witzenrath is also involved in the design, planning, and performance of the required clinical trials.
“Lung infections with antibiotic-resistant bacteria are a clinical problem of increasing relevance. We hope to be able to help patients by means of phage-based therapies in the future,” says Witzenrath. The clinical trial will be conducted in the research unit of Charité Research Organisation GmbH (CRO). Furthermore, CRO will provide organizational and regulatory assistance for the entire project, will keep close contact with supervisory drug agencies, and will take care of data management, statistics, and preparation of the clinical study report.
Dr. Andreas Hüser, senior project manager: “Charité Research Organisation has specialized in such studies and we are were excited to be part of this important project. The combination of partners in this project is truly outstanding.”
Scientific contact:
Fraunhofer ITEM
Dr. Holger Ziehr
Pharmaceutical Biotechnology
Phone +49 531 6181-6001
holger.ziehr@item.fraunhofer.de
Press contact:
Fraunhofer ITEM
Dr. Cathrin Nastevska
Phone +49 511 5350-225
cathrin.nastevska@item.fraunhofer.de
For further information and an image, please refer to:
https://www.item.fraunhofer.de/en/press-and-media/press-releases/pm-fraunhofer-i…
Further scientific contacts:
Leibniz Institute DSMZ – German Collection of Microorganisms and Cell Cultures
Dr. Christine Rohde (Bioeconomics and Health, Working Group on Phages)
Phone +49 531 2616-220
chr@dsmz.de
Charité – Universitätsmedizin Berlin
Prof. Dr. Martin Witzenrath (Medical Dep., Division of Infectiology and Pneumonology)
Phone +49 30 450553122
martin.witzenrath@charite.de
Charité Research Organisation GmbH
Dr. Andreas Hüser (Project manager)
Phone +49 30 450539231
andreas.hueser@charite-research.org
Short profiles of the project partners:
Leibniz Institute DSMZ
With over 50,000 cultures of a broad range of microorganisms and cell lines, Leibniz Institute DSMZ is one of the largest bioresource centers worldwide. Its newly founded Department of Bioeconomics and Health is committed to enhancing the phage collection and is working on a variety of research projects on phages.
Fraunhofer ITEM
The Fraunhofer ITEM Division of Pharmaceutical Biotechnology develops processes for biotechnological agents, with a focus on pharmaceutical applications. For over 20 years, active ingredients from a broad range of substance classes have been manufactured for clinical trials in the division’s laboratories.
Charité – Universitätsmedizin Berlin
Charité’s Medical Department, Division of Infectiology and Pneumonology is intensely dedicated to basic research, besides university-based healthcare and education. The working group headed by Professor Martin Witzenrath is exploring rational foundations for novel therapeutic perspectives and has specialized in translational evaluation of novel therapies. One of its numerous cooperation partners is Leibniz Institute DSMZ. For several years already, the two institutions have collaboratively studied the processes taking place during treatment of bacterial infections with phages.
Charité Research Organisation, Berlin (CRO)
CRO is a wholly-owned subsidiary of Charité – Universitätsmedizin Berlin. The contract research organization is specialized in scientifically demanding and complex phase-I and phase-II trials, which CRO has conducted in its own research unit for over 10 years.
Media Contact
More Information:
http://www.fraunhofer.deAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















