Biologists build better software, beat path to viral knowledge
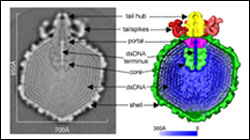
Pictured are images of Epsilon 15, a virus that infects the bacterium Salmonella. From the left-side cross section of the viral particle’s interior, obtained with an advanced magnifier called a cryo-electron microscope, a team including Purdue structural biologist Wen Jiang was able to generate the right-side computer graphic highlighting the salient features of the virus. Scientists have had difficulty resolving the internal features of viruses with non-symmetric components such as Epsilon 15, but Jiang’s team made improvements to the computer software used to process the electron microscopy images, an advance that should make many other such viruses available for medical researchers to study. (Graphic courtesy of Nature magazine/Jiang Laboratories)
Insight into the workings of previously inscrutable viruses has been made possible by a team of biologists whose improvements to computer software may one day contribute to the fight against viral disease.
With a few deft lines of computer code, Purdue University’s Wen Jiang and his research group have created a powerful new tool for lab research that should allow scientists to obtain high-resolution images of some of the world’s smallest biological entities — the viruses. Too minuscule to be usefully observed with many conventional imaging devices, viruses’ internal structures must often be viewed with microscopes that require sophisticated computer control to make sense of the tiny objects. Advances in the field often come to those who can create the best custom software, and Jiang’s team has done just that, opening up for observation a group of viruses that scientists previously could not get a bead on.
As the team reports in the cover article of this week’s (Feb. 2) edition of Nature, the researchers have used their methods to examine one such virus that attacks bacteria.
“While before we could only see virus parts that were symmetric, we can now see those that have non-symmetric structures, such as portions of the one our paper focuses on, the Epsilon 15 virus that attacks salmonella,” said Jiang, who recently joined Purdue’s College of Science as an assistant professor of biology. “This software will enable a substantial expansion of what we can see and study. We remain limited to observing those viruses that are identical from one individual viral particle to the next — which, sadly, is still only a small portion of the viral species that are out there. But it is a major step forward toward our goal of seeing them all.”
Jiang conducted the work while at Baylor College of Medicine with that institution’s Juan Chang, Joanita Jakana and Wah Chiu, as well as the Massachusetts Institute of Technology’s Peter Weigele and Jonathan King.
Developing the software package enabled the team to examine the Epsilon 15 virus, a “bacteriophage” that infects the salmonella bacterium, and to resolve features as small as 9.5 angstroms across — less than a billionth of a meter. Until now, the high-resolution device, called a cryo-electron microscope, used to examine such objects could only examine the virus’s outer shell.
“Many teams were able to determine the shell’s configuration because it is a highly symmetric, regular 20-sided shape. But to do so, they essentially had to pretend the rest of the virus didn’t exist,” Jiang said. “The trouble is that its structure is a lot more complicated than that. It has a tail and an internal genome made up of strands of tightly coiled DNA that are essential to the virus’s function. We literally didn’t have the whole picture of what tools Epsilon 15 uses to infect its host.”
The newly revealed components of the viral particle possesses qualities surprising to researchers accustomed to seeing only symmetric viruses up close.
“Epsilon 15’s tail, for example, has six ’spikes’ in it, but they aren’t arranged in a neat hexagonal ring. They’re highly deviant,” Jiang said. “Because they’re so off-kilter, only two of the spikes actually grasp the shell surface. It’s probably not very exciting news to anyone who doesn’t look at these things for a living, but what it shows us is that the viral world holds many unexpected secrets, and if we’re going to unlock them, we need to see them first.”
Probing the innards of the virus also revealed that it possesses a core, the existence of which the researchers did not suspect and the function of which they can as yet only guess at. Jiang said his team suspects the core helps ease the release of the DNA coil into the bacterium, an event akin to shooting a spool of twine attached to a grappling hook across a wall at high velocity. But he said the impact of the team’s research would likely be felt more by people who have wanted a tool to look at other viruses rather than, say, doctors with salmonella patients.
“So why do this study in the first place, if all it’s doing is helping academics increase their own knowledge?” Jiang asked rhetorically. “It’s not a simple answer, but the bottom line is, you have to solve the easy problems before you can attempt the hard ones whose answers have more immediate practical use. But where we might be able to go once we’ve taken these comparatively easy steps is quite tantalizing.
“Phages, for example, are useful to know about because they attack bacteria, and bacteria are staging a worrisome comeback in human health terms because they are growing resistant to our antibiotics — sometimes faster than medicine can keep up. We need a new way to attack bacteria once they mutate, and if we can employ phages to do our work for us, it could be a great advance for medicine.”
Phages that attack bacteria are harmless to humans, Jiang said, and for each bacterial species, including those that cause human disease, nature has evolved several phages designed to infect it specifically.
“Phage therapy as an antibacterial weapon was an idea that was introduced in the early 20th century, but it fell by the wayside as antibiotics came to the fore,” Jiang said. “It is possible that as we learn more about how viruses work on the molecular level, their promise as a medical tool will finally come to fruition. Until then, software will be the key to focusing our technological eyes, and teams like ours must keep improving it.”
This work was supported in part by the National Institutes of Health and the Robert Welch Foundation.
Jiang is associated with Purdue’s Markey Center for Structural Biology, which consists of laboratories that use a combination of cryo-electron microscopy, crystallography and molecular biology to elucidate the processes of viral entry, replication and pathogenesis.
Writer: , (765) 494-2081, cboutin@purdue.edu
Source: Wen Jiang, (765) 494-4408, jiang12@purdue.edu
Purdue News Service: (765) 494-2096; purduenews@purdue.edu
Media Contact
More Information:
http://www.purdue.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…





















