Prions Rapidly “Remodel” Good Protein Into Bad
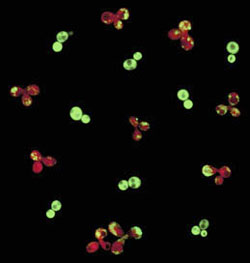
Green light/red light <br>Healthy Sup35, tagged with a green fluorescence, changed to red after converting to the infectious prion form.
Two Brown Medical School biologists have figured out the fate of healthy protein when it comes in contact with the infectious prion form in yeast: The protein converts to the prion form, rendering it infectious. In an instant, good protein goes bad.
This quick-change “mating” maneuver sheds important light on the mysterious molecular machinery behind prions, infectious proteins that cause fatal brain ailments such as mad cow disease and scrapie in animals and, in rare cases, Creutzfeldt-Jacob disease and kuru in humans.
Because similar protein self-replication occurs in neurodegenerative diseases, the findings, published in the latest issue of Nature, may also help explain the progression of Alzheimer’s, Parkinson’s and Huntington’s diseases.
Graduate student Prasanna Satpute-Krishnan and Assistant Professor Tricia Serio, both in Brown’s Department of Molecular Biology, Cell Biology and Biochemistry, conducted the research using Sup35, a yeast protein similar to the human prion protein PrP.
The researchers tagged a non-prion form of Sup35 with green fluorescent protein in one group of cells and “mated” these cells with another group that contained the prion form. When the two forms came in contact in the same cell, the green-glowing, healthy protein changed pattern – a visual sign that it converted to the prion form. These results were confirmed in a series of experiments using different biochemical and genetic techniques.
Because proteins can’t replicate like DNA and RNA – the genetic material in bacteria, viruses and other infectious agents – the research helps explain the puzzling process of how prions multiply and spread infection.
Satpute-Krishnan said the speed of protein conversion was surprising. “The prions were taking all the existing protein and refolding it immediately,” she said. “It’s a very, very rapid change.”
After the conversion, the yeast cells remained healthy but had new characteristics. This survival supports the theory that prions have endured through evolution because shape-shifting is advantageous, allowing cells to avoid stress by rapidly adjusting to a new environment.
“Our studies provide some insight into how the appearance of a misfolded protein – a rare event – can lead to devastating neurological diseases,” said Serio. “Just a small amount of prion-state protein can rapidly convert healthy protein into a pathogenic form.”
The National Cancer Institute and the Pew Scholars Program in the Biomedical Sciences funded the research.
Media Contact
More Information:
http://www.brown.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















