Computer Simulation Shows How Fibrils " Proteins That Cluster in Diseases " Form
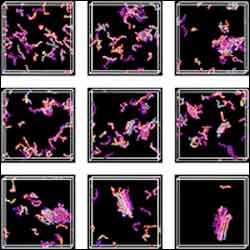
The NC State simulation shows randomly placed peptides forming a fibril.
To get a better look at how proteins gather into clusters called amyloid fibrils – which are associated with important human diseases such as Alzheimer’s, Parkinson’s and the so-called prion diseases like Mad Cow – researchers at North Carolina State University decided to make movies.
Dr. Carol Hall, Alcoa Professor of chemical engineering at NC State and Hung D. Nguyen, a graduate student in Hall’s lab, used a computer simulation technique, discontinuous molecular dynamics, to visualize the meanderings of small proteins called peptides. Movies of the simulation show that 96 randomly placed peptides spontaneously aggregate into what Hall calls a “sandwich” of layered protein sheets, similar to the amyloid fibrils discovered in diseased people and animals. Hall says that understanding how fibrils form in human or animal organs may lead to discoveries of how to slow or halt fibril formation.
The research was published in the Nov. 16 edition of Proceedings of the National Academy of Sciences. It is not known whether fibrils cause Alzheimer’s, Parkinson’s and the other so-called amyloid diseases, or whether they are just associated symptoms. In any event, the fibrils form plaques in human and animal organs, often the brain. Although it’s not clear if these plaques cause memory loss in Alzheimer’s patients, for instance, scientists are interested in finding out the mechanisms behind the formation of fibrils.
“All of these diseases – Alzheimer’s, Parkinson’s, ALS, Huntington’s – have the same unusual phenomena. Proteins – completely different proteins in each disease – assemble into ordered aggregates, amyloid fibrils, so that a vital organ, usually the brain, is crisscrossed by these structures,” Hall said. “This tells us that the problem has something to do with the general nature of proteins rather than with the specifics of the particular disease-associated proteins.”
Besides studying fibrils in the test tube, researchers would like to make computer models to view fibril formation. This is not possible using the traditional atomic-level protein folding simulation techniques – which follow the motions of every atom on every protein – because fibril formation takes a long time.
So Hall and Nguyen developed a less-detailed model of protein geometry and energetics and applied it to a relatively simple protein, polyalanine, which had been found to form fibrils in test tubes. With this approach, the NC State researchers were able to watch spontaneous fibril formation in about 60 hours on a fast computer. That’s much quicker than atomic-level simulations.
In the simulation movie, 12 to 96 peptides were initially scattered randomly across the computer screen. When set into motion, the researchers first saw groups of two to five proteins coming together and falling apart and eventually forming amorphous clumps that twist around each other, like a rope. These twisted structures began coming together, like the ingredients in a sandwich, layered above and below each other. In the end, the simulation showed a fibril-like structure with only a few outlying peptides refusing to aggregate.
Hall says her method of reducing the level of detail in her protein model just to the point where the key features that drive fibril formation remain and other features are neglected allows her to get a broad molecular-level picture of the fibril formation process.
Hall’s work is sponsored by the National Institutes of Health. She has recently been funded to attempt computer simulations of fibril formation by beta amyloids, the peptides that aggregate in Alzheimer’s disease.
Media Contact
More Information:
http://www.ncsu.eduAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles
A cause of immunodeficiency identified
After stroke and heart attack: Every year, between 250,000 and 300,000 people in Germany suffer from a stroke or heart attack. These patients suffer immune disturbances and are very frequently…

Wildfire danger to increase due to climate change
WSL Institute for Snow and Avalanche Research (SLF) researchers expect an elevated wildfire danger in the Alpine Foreland from 2040 onwards due to changing meteorological conditions. The danger currently remains…
Advanced Brain Science Without Coding Expertise
Researchers at Helmholtz Munich and the LMU University Hospital Munich introduce DELiVR, offering a new AI-based approach to the complex task of brain cell mapping. The deep learning tool democratizes…





















