Chemists make molecular interlocked rings
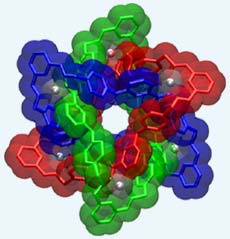
UCLA chemists have devised an elegant solution to an intricate problem at the nanoscale that stumped scientists for many years: They have made a mechanically interlocked compound whose molecules have the topology of the beloved interlocked Borromean rings. In the May 28 issue of the journal Science, the team reports nanoscience that could be described as art.
The UCLA group is the first to achieve this goal in total chemical synthesis, which research groups worldwide have been pursuing.
Named for a noble Italian family, the Borromean rings first appeared on the family’s coat of arms in the 15th century. Examples of the rings can be seen in buildings on three islands in northern Italy’s Lake Maggiore, which are still owned by the Borromeo family. The Borromean link comprises three interlocked rings that form one inseparable union such that cutting any one ring results in the other two falling apart.
“This is nanoscience, but also much more,” said Fraser Stoddart, UCLA’s Fred Kavli Professor of Nanosystems Sciences and director of the California NanoSystems Institute at UCLA. “The Borromean Rings pervade art, theology, mythology and heraldry, as well as mathematics, physics and chemistry. Go to the Google search engine and you are confronted with more than 2,000 hits.”
“The realization of the Borromean link in a wholly synthetic molecular form has long been regarded as the most ambitious and challenging target in topological chemistry — a Gordian knot,” Stoddart said. “The near-quantitative assembly of this topological link from 18 components by templation around six metals of six organic pieces with two ’teeth’ and another six with three ’teeth’ to grip the metals, resulting in the intermittent opening and closing of 12 carbon-nitrogen double bonds, cuts this Gordian knot once and for all.”
(An ancient Greek oracle foretold that whoever untied the intricate Gordian knot, a knot with no ends exposed, would rule all of Asia. The problem resisted all attempted solutions until 333 B.C., when Alexander the Great is said to have cut through the knot with his sword.)
The “high-risk, all-in-one, mix-the-pieces together, and shake-them-all-about” approach was the brain-child in November 1999 of graduate student Stuart Cantrill in Stoddart’s research group. Cantrill is now a lecturer and research associate in UCLA’s department of chemistry and biochemistry.
Aided by the computational wizardry of fellow graduate student Anthony Pease, Cantrill conceived a topology that was modeled to vindicate the perfect matching of the three identical, mutually interlocking rings around the six metal templates. “The three rings slotted into place perfectly, encompassing the six metals effortlessly in three-dimensional space,” Cantrill said.
“We both stared at the screen and agreed there and then that it just had to work,” Cantrill said. “It looked so perfect, so beautiful.”
“Putting the caboodle together in the computer was one thing; translating it into a chemical reality in the laboratory was quite another,” Stoddart said. Two of the three sets of six pieces could be bought, but the remaining one had to be made in a complex seven-step synthesis.
The first tentative steps were taken by Pease, who said, “As a computational chemist, I would normally avoid getting my hands wet in the laboratory, but this molecule was so irresistible, I decided to give it a try.”
Cantrill and Pease graduated from UCLA in 2001 and left the completion of the synthesis of the all-important third piece to postdoctoral fellow Shien-Hsien Chiu, now an assistant professor of chemistry at the National Taiwan University.
“With all three pieces in place, the most challenging part of the puzzle still lay ahead of us,” Stoddart said. “I was then blessed with the arrival in 2002 of postdoctoral researcher extraordinaire Kelly Chichak. He brought with him a knowledge base and expertise in coordination and supramolecular chemistry that made him a natural when it came to doing chemical synthesis in a proofreading, error-checking fashion. It would not have happened without Kelly’s nous.”
Chichak said, “I just happened to land in the right place at the right time. I was immediately sucked into the quest for the molecular Borromean rings because of their rich history and appealing symmetry.”
His challenge was to unearth just the right set of conditions to coax 18 components to click together in one way and give “a beautifully crafted molecule which literally made itself in my hands,” according to Chichak.
Stoddart views the near-quantitative assembly as one of the finest that dynamic chemistry has delivered in his laboratory to date. “It doesn’t happen all that often: it is good old thermodynamics to the rescue, with a real vengeance at that.”
Or as Chichak puts it, “Simply mix and heat and a single product emerges out of the thousands of possibilities: That’s all I needed to do.”
More than 30 years ago, Robert Woodward at Harvard and Albert Eschenmoser at the Swiss Federal Institute of Technology (ETH) in Zürich created Vitamin B12 chemically in a laboratory, a triumph of chemical synthesis, Stoddart noted. “Similarly, during the past decade, a total synthesis of Borromean rings in a molecular form has become the Herculean challenge in contemporary synthesis, where Darwinian selection operates in a chemically evolving system,” he said.
Chichak obtained X-ray-quality single crystals from which postdoctoral fellow Gareth Cave solved the structure in the laboratory of Jerry Atwood, chemistry professor at the University of Missouri, Columbia.
Each molecule of the Borromean ring compound is 2.5 nanometers across and contains an inner chamber that is a quarter of a cubic nanometer in volume and is lined by 12 oxygen atoms.
“When your mind turns to potential applications, the molecule has so much going for it,” Stoddart said.
“Now that we are addressing what they might do for us, the list becomes endless,” Chichak said.
Release URL, if available: The URL must point to the specific release, not a general page of releases or your organization’s main homepage.IThe ability to produce gram quantities of highly soluble hosts that can locate a range of different transition metals in an insulated octahedral array around an inner oxygen atom-lined chamber, which can provide a welcoming home for many different guests, suggests numerous ways in which these molecular Borromean rings could be explored as highly organized nano?clusters in a materials setting such as spintronics or in a biological context such as medical imaging, Stoddart said.
“When all is said and done, the molecular Borromean rings should be judged by their magnificent looks at this early stage in their existence,” Stoddart said. “As one of the reviewers of the original manuscript wrote, ’The beauty of the molecular structure is really breathtaking.’”
Media Contact
More Information:
http://www.ucla.edu/All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Peptides on Interstellar Ice
A research team led by Dr Serge Krasnokutski from the Astrophysics Laboratory at the Max Planck Institute for Astronomy at the University of Jena had already demonstrated that simple peptides…

A new look at the consequences of light pollution
GAME 2024 begins its experiments in eight countries. Can artificial light at night harm marine algae and impair their important functions for coastal ecosystems? This year’s project of the training…

Silicon Carbide Innovation Alliance to drive industrial-scale semiconductor work
Known for its ability to withstand extreme environments and high voltages, silicon carbide (SiC) is a semiconducting material made up of silicon and carbon atoms arranged into crystals that is…





















