New microscope technology allows study of biomolecules interacting with minerals

Virginia Tech student presents first findings at international geochemistry conference
Every living thing needs iron. The strategies some organisms use to accumulate iron can impact the quality of our environment and could be adapted for our use.
Imagine a falconer releasing his falcon to nab pigeons for his dinner. That is somewhat how the bacteria, Azotobacter vinelandii, acquire iron. They release siderophore molecules, called azotobactin, which nabs iron out of minerals.
“The molecule extracts the iron from the mineral and is expected to eventually return with the iron to the bacterial cell,” says Treavor Kendall, a Ph.D. candidate in the mineral-microbe group in Virginia Tech’s Department of Geological Sciences.
Azotobacter vinelandiihas two things going for it. It releases millions of siderophores and these molecules have “a huge affinity for iron — some of the highest affinities observed in nature,” says Kendall.
Kendall studies how bacteria acquire iron. There have been a lot of studies on siderophores in the aqueous phase. “We do know how siderophores behave with iron in water,” Kendall states, “But we don’t know how they interact with iron that is locked up in a mineral structure. This is important because minerals are a primary source of iron in the environment.”
Kendall’s research is looking specifically at the affinity or forces between azotobactin and the mineral goethite — an important iron oxide in soils worldwide.
He has been invited to present his research at the 12th Annual V.M. Goldschmidt Conference, an international geochemistry conference, Aug. 18-23, 2002 in Davos, Switzerland. His paper will be presented Thursday morning, Aug. 22, during the symposium on “Biogenic substances and their effect on trace metal cycling and mineral weathering” (S36 Wednesday p.m. and Thursday a.m.).
Kendall explains that when the molecule removes the iron from the mineral, it actually dissolves the mineral. “What happens if that mineral also contains lead or some other toxic metal? The siderophore can knock off those toxic metals, which then pollute the fresh water, marine environment, or semi-humid soil where these interactions most frequently occur.”
Kendall has attached a siderophore to the microscopic plank or cantilever used in an atomic force microscope (AFM). The siderophore molecule is lowered toward the mineral surface to measure how it interacts. “The attraction is so high, that the cantilever actually snaps down to attach the molecule to the mineral,” Kendall says. “When we pull it apart, like lifting your shoe off hot gum on the sidewalk, the molecule actually stretches until it breaks loose.
“Based on how much the molecule sticks, we can comment on how well siderophore likes that surface,” says Kendall.
“The excitement is being able to measure the affinity between the siderophore molecule and the iron in the mineral structure.”
At the conference, Kendall will report on three experiments.
“First, we are able to measure forces between the siderophore molecule and the goethite, and compare that with how siderophore interacts with diaspore, a mineral that contains aluminum. It works out as you would expect,” says Kendall. “There is a higher affinity with the iron mineral.
“Next, we introduce a soluble or free form of iron. All of a sudden, the affinity goes away. This tells us that the siderophore is satisfied and no longer needs the iron in the mineral. It confirms that we are measuring what we thought,” says Kendall. “So we have demonstrated the relationships and how we can make it go away.
“Third we demonstrate that the relationship doesn’t change when we alter the solution by changing the pH and ionic strength,” says Kendall. “Thus, we are confident that we are measuring a specific interaction.”
Potential environmental applications include anticipating toxic metal release and studying iron availability in soils.
Presently, siderophores are used in medicine to treat people who have too much iron in their blood. The siderophore locks up the iron so it is no longer toxic. The ability to measure iron affinity at the molecular level may allow researchers to refine siderophore medicinal use and detect iron concentrations in very small amounts by using them as a chemosensor. There has already been a paper exploring siderophores as chemosensors by other researchers, Kendall says.
Kendall’s major professor is Michael Hochella. Research funding is provided by the U.S. Department of Education, Kendall’s GAAN fellowship, the National Science Foundation, and the Department of Energy. The talk in Switzerland is Kendall’s first invited talk.
Originally from Houston, Kendall did his undergraduate work at the University of Texas at Austin and his master’s degree work at the University of Montana, Missoula.
Reach Kendall at tkendall@vt.edu or 540.231.8575.
He is in the lab most days from 8 a.m. to 7p.m. He leaves Aug. 17, but will be available by e-mail while in Switzerland.
Learn more about the Goldschmidt Conference at http://www.goldschmidt-conference.com/2002/gold2002/
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles
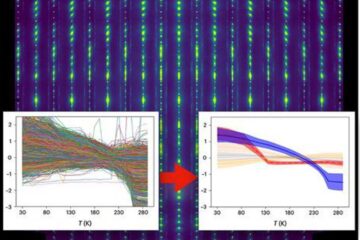
Machine learning algorithm reveals long-theorized glass phase in crystal
Scientists have found evidence of an elusive, glassy phase of matter that emerges when a crystal’s perfect internal pattern is disrupted. X-ray technology and machine learning converge to shed light…
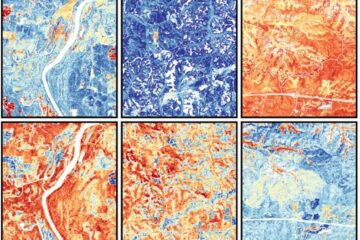
Mapping plant functional diversity from space
HKU ecologists revolutionize ecosystem monitoring with novel field-satellite integration. An international team of researchers, led by Professor Jin WU from the School of Biological Sciences at The University of Hong…

Inverters with constant full load capability
…enable an increase in the performance of electric drives. Overheating components significantly limit the performance of drivetrains in electric vehicles. Inverters in particular are subject to a high thermal load,…





















