Protein shows talent for improvisation
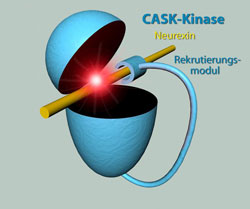
A protein with two functions: Using a clever trick, the CASK kinase compensates - at least in part - for its low activity. One part of the protein actively recruits neurexin proteins and places them in close proximity to the kinase. Wahl / MPIbpc<br>
Radio and cable are not required for communication within and between living cells. Rather, signal transduction in cells is performed by a multitude of proteins.
In order to transfer and interpret these signals correctly, activities of these proteins have to be precisely synchronized. Their subtle regulation is controlled by a sophisticated system, in which so called protein kinases play a key role. An international team of scientists from Dallas (USA), Göttingen and Hamburg (Germany) have now discovered a kinase, which seems superior under difficult conditions. Whereas all known kinases function only in the presence of magnesium, the pseudokinase CASK has found a trick to do away with this trace element.
The protein seems to be directly involved in formation of contact sites – synapses – during early development of the nervous system. Pseudokinases like CASK have so far been considered inactive. At least some of them seem to have been labelled “not useful” without good reason in the past. (Cell, April 18, 2008)
Human beings must permanently adjust to new situations in their environment and react in an appropriate manner. Likewise, living cells receive a large number of signals which they need to transfer and to interpret. Often, cells are stimulated to grow or to divide, to start a developmental process or to initiate an immune response. To do so, numerous actors within cells – the proteins – have to perform in a precisely coordinated manner. A complex control system assures that these proteins work at the right time and at the right place. Central key players within this control system are specific proteins termed kinases. Up to 500 different kinases are present within a single cell; each of them regulates a particular subset of proteins. They activate or inhibit proteins, route them to a specific cellular location, or block their interaction with other cell components. To transmit their orders, kinases label corresponding proteins with a small phosphate group. The underlying reaction mechanism seems to be the same for all known kinases: With the help of magnesium, kinases bind an ATP-molecule and cleave off one phosphate group, which is subsequently transferred to the protein. A small number of kinases, however, lack the ability to bind magnesium normally required for the reaction. As so-called “pseudokinases” they have so far been largely disregarded in research. Wrongfully, as shown now by an international team of scientists of the University of Texas (Dallas, USA), the Max Planck Institute for Biophysical Chemistry (Göttingen, Germany) and the Deutsches Elektronen Synchrotron (Hamburg, Germany).
The researchers investigated a pseudokinase – the CASK kinase – which seems to be actively involved in early development of the nervous system. CASK interacts directly with the protein neurexin, which is required for correct formation of synapses between nerve cells. Mice lacking CASK kinase die shortly after birth. Humans without CASK develop mental disorders and blindness. “But CASK can not bind magnesium and without magnesium kinases usually do not work. For us, this just did not add up”, says neurobiologist Konark Mukherjee, one of the project leaders of the University of Texas. Therefore, the scientists simulated the reaction in the test tube step by step. To their surprise the CASK kinase transferred phosphate groups completely without magnesium. When the scientists added magnesium to the test tube, the kinase was in fact inhibited. But is CASK also functional in a living cell? Indeed, the researchers could prove that the kinase performs in the same way in nerve cells of rats. In biological terms, the improvised reaction mechanism of CASK makes perfect sense. During synapse formation nerve cells contain little to no magnesium. Kinases, which depend on magnesium for function would simply not be functional”, explains Mukherjee.
One protein – two functions
One exciting question for the scientists is now how a kinase can also do its job without magnesium. To better understand this novel reaction mechanism, neurobiologists and structural biologists worked closely together. Using X-ray crystallography, the scientists successfully solved the atomic structure of the CASK kinase. “In contrast to classic kinases CASK is virtually permanently active. But it reacts much slower in contrast to magnesium-dependent kinases”, summarizes structural biologist Markus Wahl of the Max Planck Institute for Biophysical Chemistry the new insights into CASK function. The protein compensates its low activity – at least in part – by a clever trick: Besides the kinase domain, the protein contains another part, which actively recruits neurexin proteins and therefore facilitates their reaction with the kinase domain. “This way the pseudokinase can interact with the neurexin substrate for a longer time and label it with phosphate groups, although it acts slowly”, explains Markus Wahl. The results of the researchers show that the reaction mechanism of kinases is much more multi-faceted than presumed earlier. Similarly, other pseudokinases, which lack typical features of kinases, could emerge as “specialists” which are functional under conditions where classical kinases would fail.
Contact:
Dr. Markus Wahl
Max Planck Institute for Biophysical Chemistry
Phone: +49 551 201-1046
Fax: +49 551 201-1197
E-Mail: mwahl@gwdg.de
Dr. Konark Mukherjee
University of Texas Southwestern Medical Center
Phone: +1 214-648-1903
Fax: +1 214-648-1801
E-Mail: konark.mukherjee@utsouthwestern.edu
Dr. Carmen Rotte
Public relations office, Max Planck Institute for Biophysical Chemistry
Phone: +49 551 201-1304
Fax: +49 551 201-1151
E-Mail: pr@mpibpc.mpg.de
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















