Protein “filmed” while unfolding at atomic resolution
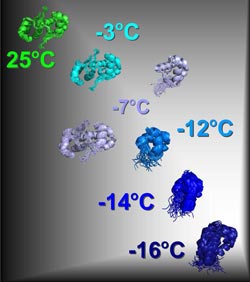
“Snapshot” of the unfolding of the CylR2 protein from Enterococcus faecalis. If the protein is cooled from 25°C to -16°C, it successively breaks down into its two identical subunits. The latter are initially stable, but at -16°C they form an instable, dynamic protein form, which plays a key role in folding.<br><br>© Zweckstetter, Max Planck Institute for Biophysical Chemistry & German Center for Neurodegenerative Diseases <br>
Researchers at the Max Planck Institute for Biophysical Chemistry and the German Center for Neurodegenerative Diseases in Göttingen – in collaboration with Polish colleagues – have now “filmed” how a protein gradually unfolds for the first time.
By combining low temperatures and NMR spectroscopy, the scientists visualized seven intermediate forms of the CylR2 protein while cooling it down from 25°C to – 16°C. Their results show that the most instable intermediate form plays a key role in protein folding. The scientists’ findings may contribute to a better understanding of how proteins adopt their structure and misfold during illness. (Nature Chemical Biology, 10. February 2013)
Whether Alzheimer’s, Parkinson’s or Huntington’s Chorea – all three diseases have one thing in common: They are caused by misfolded proteins that form insoluble clumps in the brains of affected patients and, finally, destroy their nerve cells. One of the most important questions in the biological sciences and medicine is thus: How do proteins – the tools of living cells – achieve or lose their three-dimensional structure. Because only if their amino acid chains are correctly folded, can proteins perform their tasks properly.
But what exactly happens when proteins fold or unfold was previously nearly impossible to investigate. With heat and pressure, proteins easily lose their shape – and thus their function. However, such methods are not suitable for directly observing their unfolding process. The intermediate forms that occur in the course of protein folding are much too transient.
With a novel approach, researchers have now succeeded in “filming” the complex process of protein folding for the first time. Scientists at the Max Planck Institute for Biophysical Chemistry (MPIbpc) and the German Center for Neurodegenerative Diseases (DZNE) in Göttingen, together with their colleagues at the Polish Academy of Sciences in Warsaw and at the University of Warsaw, have rendered visible – at atomic resolution – how a protein progressively “loses its shape”.
In doing so, the researchers had pinned their hopes on low temperatures. “If a protein is slowly cooled down, its intermediate forms accumulate in larger quantities than in commonly used denaturation methods, such as heat, pressure, or urea. We hoped that these quantities would be sufficient to examine the intermediate forms with nuclear magnetic resonance (NMR) spectroscopy,” said Markus Zweckstetter, head of the research groups “Protein Structure Determination using MNR” at the MPIbpc and “Structural Biology in Dementia” at the DZNE in Göttingen.
How a protein loses its shape
As research object, Zweckstetter’s team chose a key protein for toxin production in Enterococcus faecalis, a pathogen frequently encountered in hospitals where it particularly jeopardizes patients with a weak immune system. But that is not the only reason why the so-called CylR2 protein is interesting. Some time ago, researchers working with Stefan Becker at the MPIbpc succeeded in elucidating its structure, which shows: Its three-dimensional shape makes CylR2 a particular promising candidate for the scientists’ approach. “ClyR2 is a relatively small protein composed of two identical subunits. This gave us a great chance to be able to visualize the individual stages of its unfolding process in the test tube,” explained the chemists Mariusz and Lukasz Jaremko.
Stefan Becker's group undertook the first step: to prepare a sufficient quantity of the protein in the laboratory. Subsequently, the two chemists cooled the protein successively from 25°C to -16°C and examined its intermediate forms with NMR spectroscopy. They achieved what they had hoped for: Their “film clip” shows at atomic resolution how the protein gradually unfolds. The structural biologist Markus Zweckstetter describes exactly what happens in this process: “We clearly see how the CylR2 protein ultimately splits into its two subunits. The individual subunit is initially relatively stable. With further cooling, the protein continues to unfold and at -16 °C it is extremely instable and dynamic. This instable protein form provides the seed for folding and can also be the “trigger” for misfolding.” The scientist’s findings may help to gain deeper insights into how proteins assume their spatial structure and why intermediate forms of certain proteins misfold in the event of illness. (cr)
Original Publication
Mariusz Jaremko, Lukasz Jaremko, Hai-Young Kim, Min-Kyu Cho, Charles D. Schwieters, Karin Giller, Stefan Becker, Markus Zweckstetter
Cold-denaturation of a protein dimer monitored at atomic resolution.
Nature Chemical Biology, DOI:10.1038/NChemBio.1181 (2013)
Contact
Prof. Dr. Markus Zweckstetter
Protein Structure Determination using NMR
Phone:+49 551 201-2220
Email: mzwecks@gwdg.de
Dr. Carmen Rotte
Press Officer, Public Relations Office
Phone:+49 551 201-1304Fax:+49 551 201-1151
Email: carmen.rotte@mpibpc.mpg.de
Dr. Dirk Förger
Head of Press and Public Relations, German Center for Neurodegenerative Diseases Bonn
Phone:+49 228 43302-260
Email: dirk.foerger@dzne.de
Media Contact
More Information:
http://www.mpibpc.mpg.de/9606319/pr_1302All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Making diamonds at ambient pressure
Scientists develop novel liquid metal alloy system to synthesize diamond under moderate conditions. Did you know that 99% of synthetic diamonds are currently produced using high-pressure and high-temperature (HPHT) methods?[2]…

Eruption of mega-magnetic star lights up nearby galaxy
Thanks to ESA satellites, an international team including UNIGE researchers has detected a giant eruption coming from a magnetar, an extremely magnetic neutron star. While ESA’s satellite INTEGRAL was observing…

Solving the riddle of the sphingolipids in coronary artery disease
Weill Cornell Medicine investigators have uncovered a way to unleash in blood vessels the protective effects of a type of fat-related molecule known as a sphingolipid, suggesting a promising new…





















