Proof of water wires motivated by a biological water channel
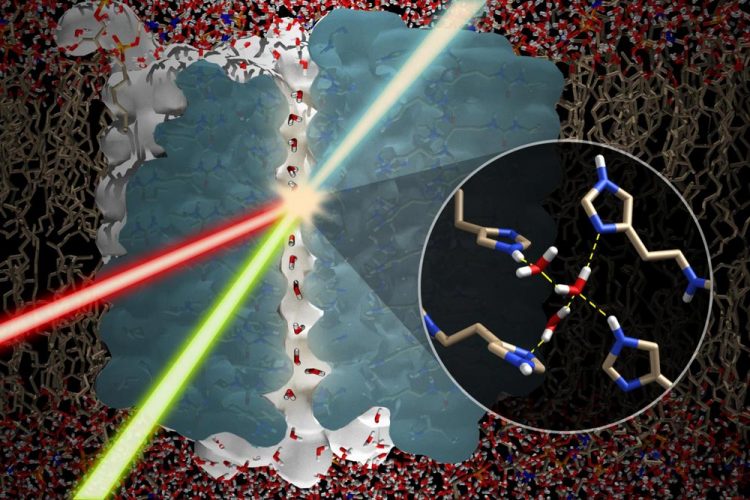
Details of a water wire (central channel) embedded in a self-assembled immidazole channel (blue) in a lipid-bilayer. The summation of the incident visible (green) and IR (red) beams produce a resulting SFG (sum frequency generation) beam that characterizes the hydrogen bonding of the water wire at the molecular level (courtesy of Poul Petersen, Cornell University). Credit: Rensselaer Polytechnic Institute
Aquaporins are proteins that serve as water channels to regulate the flow of water across biological cell membranes. They also remove excess salt and impurities in the body, and it is this aspect that has led to much interest in recent years in how to mimic the biochemical processes of aquaporins potentially for water desalination systems.
An international team of researchers co-led by Georges Belfort has discovered water, in the form of “water wires,” contained in another molecule–the imidazole–a nitrogen-based organic compound that could be used as a potential building block for artificial aquaporins. The findings were recently published in Science Advances by the American Association for the Advancement of Science. Belfort is Institute Professor and professor of chemical and biological engineering at Rensselaer Polytechnic Institute.
Belfort's colleague, Mihail Barboiu, a research leader at the European Membranes Institute (EMI) in France, has synthesized and studied the dynamics of a ring structure of the imidazole embedded in a supported lipid bilayer (i.e., in a synthetic model of a biological membrane surrounding a cell). EMI operates under the auspices of several organizations, including France's National Center for Scientific Research (abbreviated CNRS in French).
X-ray studies by Barboiu and dynamic computer simulations by CNRS researcher Marc Baaden show that the imidazole's ring structure makes the molecule an ideal candidate to learn about how artificial aquaporins could be developed. In theory, assembled imidazole molecules act like an aquaporin by allowing water molecules to enter and possibly flow through the center of the ring structure while keeping out other molecules.
Still, there was no direct proof that water existed inside the imidazole water channel. To find out, Barboiu enlisted the help of Belfort and Poul Petersen, assistant professor of chemistry at Cornell University.
Through their experimental studies, Belfort and Petersen have found that not only does water exist in the imidazole water channel, but also that the imidazole ring construct induces the water molecules to self-assemble into a highly oriented linear chain structure–or what the researchers have dubbed “water wires.”
“For the first time, we have made a direct observation of this unique water structure inside a synthetic water channel that mimics an aquaporin,” Belfort said.
Belfort and his colleagues also discovered that the chirality of the imidazole molecules orients the water molecules and could increase the permeability of water through the water channel compared with achiral (i.e., not chiral) imidazole molecules that they also assembled. Chirality happens when a mirror image of an object is not superimposable–for example, your left and right hand.
In the case of the imidazole molecule, its chirality depends on the way that the groups of atoms in a molecule are organized. As Belfort explained, the chiral imidazole atoms can be seen as spokes on a bicycle wheel that cannot be superimposed on the “spokes” of an imidazole that is achiral.
“If you place several of these rings on top of each other like a pile of pancakes, the center (the 'axel') of the spokes holds the water molecules and enables them to connect with one another in an ordered way to form a water wire,” he said. “Our results also showed that the water wire changed its orientation when imidazole chirality changes, further confirming that the chiral shape of the imidazole controls how the water behaves.”
In their study, the researchers used artificial water channels that they created from imidazole self-assembled structures inside lipid bilayers, thin membranes that form a continuous barrier around cells. The imidazole building blocks were synthesized by Barboiu and his group in France. Belfort's research group then assembled the lipid bilayers to contain the imidazole structures.
Belfort's team used a quartz crystal microbalance (QCM) to measure the assembly and water content. Researchers use QCM to measure small mass changes on a vibrating quartz crystal. The lipids containing the water wire structures were then carried to Cornell by Mirco Sorci, a research associate in Belfort's lab, to further analyze the presence of the water wire and its orientation, using a special instrument that measures hydrogen bonds between water molecules called a sum frequency generation spectrometer.
###
About Rensselaer Polytechnic Institute
Rensselaer Polytechnic Institute, founded in 1824, is America's first technological research university. For nearly 200 years, Rensselaer has been defining the scientific and technological advances of our world. Rensselaer faculty and alumni represent 86 members of the National Academy of Engineering, 17 members of the National Academy of Sciences, 25 members of the American Academy of Arts and Sciences, 8 members of the National Academy of Medicine, 8 members of the National Academy of Inventors, and 5 members of the National Inventors Hall of Fame, as well as 6 National Medal of Technology winners, 5 National Medal of Science winners, and a Nobel Prize winner in Physics. With 7,000 students and nearly 100,000 living alumni, Rensselaer is addressing the global challenges facing the 21st century–to change lives, to advance society, and to change the world. To learn more, go to http://www.
Contact: newsmedia@rpi.edu
Visit the Rensselaer research and discovery blog: http://approach.
Follow us on Twitter: http://www.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…





















