Princeton researchers explore how a carbon-fixing organelle forms via phase separation
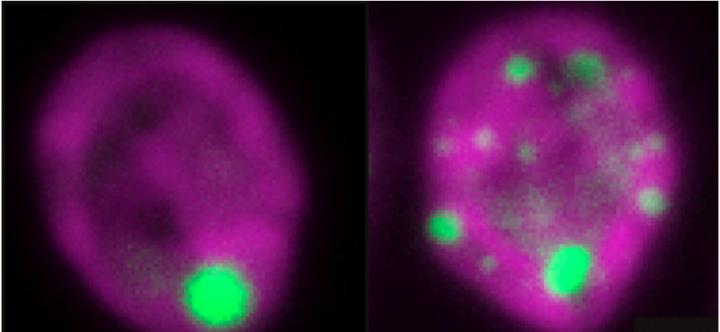
The left panel shows a type of single-celled algae known as Chlamydomonas reinhardtii with its chloroplast (purple) containing only a single pyrenoid (green). The SAGA1 mutant shown on the right displays several extra pyrenoids. Credit: Alan Itakura
Plants, algae and other photosynthetic organisms remove carbon dioxide from the air, incorporating it into starches in a process known as carbon fixation. In green algae, which contribute up to a third of global carbon fixation, this activity is greatly enhanced by an organelle called the pyrenoid.
A new paper by Princeton researcher Martin Jonikas, assistant professor of molecular biology, and colleagues, which appeared online in the journal Proceedings of the National Academy of Sciences on August 27, 2019, investigates a gene important for regulating pyrenoid shape and number, and enhances our understanding of this essential component of the global carbon cycle.
In algae, as in plants, the task of carbon fixation is carried out by an enzyme known as Rubisco within the chloroplast, the cellular compartment where photosynthesis takes place. In plants, Rubisco occurs throughout the chloroplast, but in algae, Rubisco molecules cluster together to form a distinct structure, the pyrenoid.
This structure assembles via a process known as phase separation in a manner similar to how oil forms clusters when placed in water. The pyrenoid is surrounded by a starch-based sheath. In most species, the pyrenoid also contains tubules that extend into it from the thylakoid, where light-dependent reactions of photosynthesis occur. Thylakoid tubules convey concentrated carbon dioxide to Rubisco, greatly improving the enzyme's efficiency–something that is very important for algae since they live in aquatic environments where carbon dioxide can be hard to access.
Prior studies by Jonikas' group have shown that the pyrenoid is not a permanent feature of the cell, but instead dissolves during cell division then re-forms as small clusters of phase-separated proteins that coalesce into a larger mass. Algal chloroplasts normally have only one pyrenoid because, like oil poured onto water, phase-separated protein clusters aggregate together to minimize their exposed surface area.
While conducting a screen for genes affecting pyrenoid function in the green alga Chlamydomonas reinhardtii, graduate student and the study's first author Alan Itakura and postdoctoral researcher Leif Pallesen, both in the Jonikas group, uncovered a gene called SAGA1 (Starch Granules Abnormal-1), the loss of which causes cells to grow poorly. When the researchers, including the study's co-first author, Kher Xing (Cindy) Chan in Howard Griffiths' group at the University of Cambridge, examined the mutant cells, they noticed that SAGA1 mutants possess multiple pyrenoids–up to 10 per cell. This was surprising since normal cells almost always contain just one pyrenoid. Intrigued, the team decided to investigate further.
Because the SAGA1 protein is predicted to contain a starch-binding domain, the researchers first explored whether loss of the SAGA1 gene affects the architecture of the starch plates that make up the pyrenoid sheath. Indeed, the pyrenoids in SAGA1-deficient cells have fewer and abnormally elongated starch plates in their sheaths. The authors also found evidence that the SAGA1 protein binds to Rubisco. Together, these data suggest that SAGA1 helps direct the proper formation of the pyrenoid's starch sheath, and the attachment of Rubisco to it.
But why would loss of SAGA1 affect pyrenoid number? The study results suggest that the increased surface area of the defective starch sheaths lead to the formation of multiple pyrenoids. Normally, the starch plates are sized appropriately to create a single pyrenoid, but the elongated starch plates in SAGA1 mutants pinch off portions of the matrix, resulting in extra pyrenoids.
Although this model explains why more pyrenoids might appear in SAGA1 mutants, it doesn't explain why excess pyrenoids hamper cell growth. The authors found that Rubisco levels are unchanged in SAGA1 mutants, suggesting that the same amount of protein is distributed across multiple pyrenoids. However, the researchers noticed that most of these extra pyrenoids lack a thylakoid tubule network. Pyrenoids without a thylakoid network would be starved of carbon dioxide, suggesting the Rubisco they contain is idled and not contributing to growth.
The work provides a useful new model to explain how a peripheral component, the starch sheath, helps cells regulate their number of pyrenoids. The authors suggest that such a mechanism may also apply to the biogenesis of other phase-separated organelles such as stress granules.
###
The research involved contributions from researchers at the Carnegie Institution for Science, Stanford University, the University of Edinburgh, the University of Cambridge and Washington University in St. Louis.
This study was supported by grants from the National Science Foundation, the National Institutes of Health, the Simons Foundation, the Howard Hughes Medical Institute, the Biotechnology and Biological Sciences Research Council, the Cambridge IBD International Scholarship, the Cambridge Philosophical Society, the Cambridge Trust, the Lundgren Fund and the Houston Putnam Lowry Prize (Fitzwilliam College).
Citation: Alan Itakura, Kher Xing Chan, Nicky Atkinson, Leif Pallesen, Lianyong Wang, Gregory Reeves, Weronika Patena, Oliver Caspari, Robyn Roth, Ursula Goodenough, Alistair McCormick, Howard Griffiths, Martin Jonikas. A Rubisco-binding protein is required for normal pyrenoid number, and starch sheath morphology in Chlamydomonas reinhardtii. Proceedings of the National Academy of Sciences, first published online on August 27, 2019.
Media Contact
More Information:
http://dx.doi.org/10.1073/pnas.1904587116All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…





















