Painkiller by Photosynthesis
<br>
During photosynthesis, plants capture solar energy and use it to drive chemical reactions. Their carbon source is the CO2 in air. In the journal Angewandte Chemie, American scientists have now proposed a new reaction mechanism that binds CO2 and strongly resembles photosynthesis.
In this process, light energy is captured by silicon nanowires. It was successfully used to synthesize two precursors of the anti-inflammatory, pain reducing drugs ibuprofen and naproxen.
Natural photosynthesis involves two processes, the light and dark reactions. In the light reactions, photons are captured and their energy stored in the form of chemical compounds like NADPH (nicotinamide adenine dinucleotide phosphate) and ATP (adenosine triphosphate), which subsequently are used to bind CO2 for the synthesis of complex sugar molecules. At the heart of the dark reactions is the binding of CO2 to a sugar phosphate (ribulose-1,5-bisphosphate). This results in formation of a â–keto acid, which gets converted to a central building block for sugar synthesis.
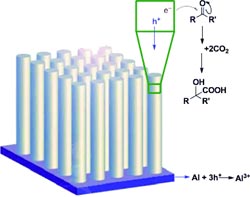
A team led by Kian L. Tan and Dunwei Wang at Boston College (Chestnut Hill, USA) has been inspired by the mechanisms of the dark reactions. To capture sunlight, the scientists used p-doped silicon nanowires as a photocathode. These very effectively convert solar energy to electrical energy, are easy to produce, and are amazingly stable under the reaction conditions needed. Captured photons release electrons from the atoms in the nanowires.
These electrons can easily be transferred to organic molecules to trigger chemical reactions. The researchers chose aromatic ketones as their starting materials. Transfer of electrons from the photocathode “activates” these molecules so that they can attack and bind CO2. Over several steps, the reaction produces an á-hydroxy acid.
This allowed the team to produce precursors of ibuprofen and naproxen with high selectivity and in high yield.
This reaction sequence closely resembles natural photosynthesis and is completely different from previous approaches to binding CO2 with the aid of sunlight. This finally solves a problem: The very poor selectivity that automatically accompanies all traditional attempts at the direct photoreduction of CO2 has limited previous methods to the production of fuels. This new strategy delivers the selectivity required for the production of complex organic intermediates for the production of pharmaceuticals and high-value fine chemicals.
About the Author
Dr Dunwei Wang, recently promoted to the rank of Associate Professor of Chemistry, researches in the area of materials chemistry at Boston College. He is interested in synthesizing and understanding new semiconducting materials for more efficient solar energy harvesting and storage.
Author: Dunwei Wang, Boston College, Chestnut Hill (USA), https://www2.bc.edu/~dwang/
Title: Silicon Nanowires as Photoelectrodes for Carbon Dioxide Fixation
Angewandte Chemie International Edition, Permalink to the article: http://dx.doi.org/10.1002/anie.201202569
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…





















