On the way to a biological alternative
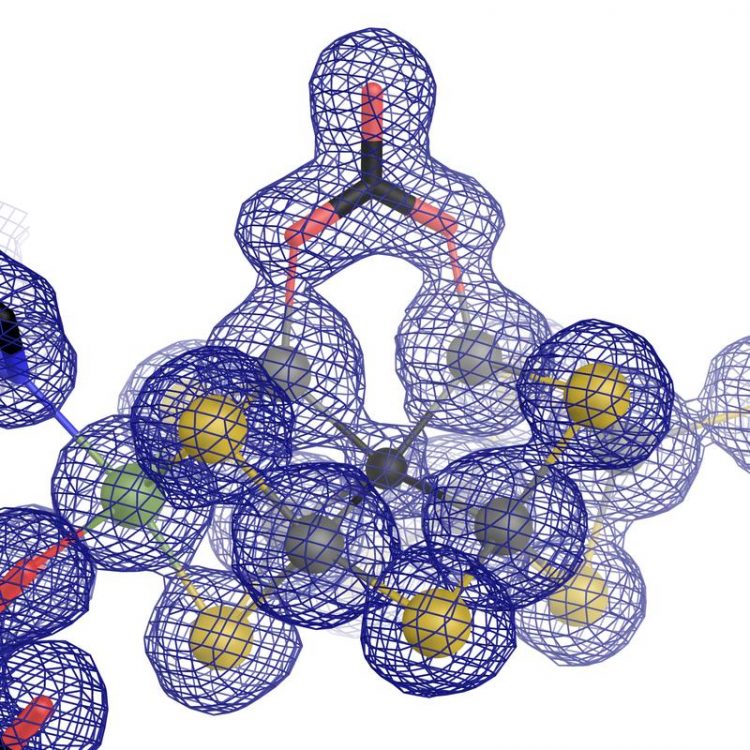
The catalytic center of vanadium nitrogenase: an iron-vanadium cofactor with an unusual carbonate ligand. Graphic: Oliver Einsle
The research team of Prof. Dr. Oliver Einsle at the University of Freiburg's Institute of Biochemistry has long been exploring the functioning of nitrogenase. Now the group is introducing the first three-dimensional structural analysis of the enzyme variant that contains vanadium.
Within the scope of preparing his doctoral thesis, Daniel Sippel succeeded in producing and crystallizing vanadium nitrogenase. Taking this as his basis, he used x-ray diffraction experiments to elucidate its spatial structure at the level of atomic resolution.
The team's long term goal is to make nitrogenase biotechnologcially useful in order to develop alternatives to industrial chemical processes. The researchers have presented their findings in the scientific journal “Nature Chemical Biology.”
The element nitrogen (N) is a key component of all organic macromolecules. Its availability in the biosphere is limited by the fact that the global occurrence of nitrogen is confined largely to the gas N2 in the atmosphere. The stability of N2 furthermore makes it inaccessible to almost all organisms. Biologically available nitrogen for agricultural fertilizer has been made since 1906 using the Haber-Bosch process.
This industrial process converts atmospheric nitrogen (N2) to ammonia through a reaction with hydrogen. Its significance is so important today because food production for more than half of the world's people can only be guaranteed with the aid of nitrogen fertilizers.
In nature, only one enzyme – bacterial nitrogenase – can achieve the same reaction, but without emitting excess nitrogen compounds into the environment, or in other words, leaching of nitrates into groundwater. Yet until now, the function of this complex, metal-containing enzyme system which contains metal has only been partially explained.
Einsle's team has already taken a significant step towards greater understanding of nitrogenase. The researchers were able to inhibit the activity of the enzyme using the toxic gas carbon monoxide (CO) to show how the inhibitor binds to the iron molybdenum cofactor (FeMoco). Known as the core of nitrogenase, it has been named for the elements it contains.
FeMoco can catalyze the reaction of nitrogen and hydrogen in a natural version of the Haber-Bosch process. At the same time it was known that a variant of nitrogenase containing vanadium rather than molybdenum in its active center and therefore called FeVco can also convert carbon monoxide.
The products of this reaction are reduced carbon compounds in the form of short carbon chains. This reaction is the enzymatic version of a second significant chemical process – Fischer-Tropsch synthesis of hydrocarbons which can be used on a large scale to synthesize fuels from industrial waste gases, for instance.
Vanadium nitrogenase found in soil bacteria can in its natural setting perform the same synthesis that is only possible in industrial processes with the aid of extreme pressures and high temperatures. The Haber-Bosch and Fischer-Tropsch processes are annually used to convert hundreds of millions of tons of the respective gases – N2 and CO – making the possibility of a sustainable, biological alternative of considerable scientific interest.
During the research work, it became apparent that most parts of the architecture of the enzyme were similar to the “original” containing molybdenum. Nevertheless, there is in important distinction that sets them apart – the atomic structure of the catalytic cofactor. Sippel and Einsle found that a vanadium ion replaces the molybdenum ion in FeVco, and includes an additional replacement of a bridging sulfide ion with a chemically very different carbonate anion (µ-1,3 carbonate — bridging ligand). What initially appears to be a slight difference has far-reaching effects on the geometric and electronic structure of the cofactor.
The research is being funded by the European Research Council (ERC) and the German Research Foundation (DFG) within the framework of the research training group 1976 “Functional Diversity of Cofactors” of the University of Freiburg and the Priority Program “Iron-Sulfur for Life.”
Original publication:
Daniel Sippel & Oliver Einsle (2017): The structure of vanadium nitrogenase reveals an unusual bridging ligand. In: Nature Chemical Biology.
DOI: 10.1038/nchembio.2428
Contact:
Prof. Dr. Oliver Einsle
Institute of Biochemistry / BIOSS Centre for Biological Signalling Studies
University of Freiburg
Tel.: 0761/203-6058
E-Mail: einsle@biochemie.uni-freiburg.de
https://www.pr.uni-freiburg.de/pm-en/2017/on-the-way-to-a-biological-alternative
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Combatting disruptive ‘noise’ in quantum communication
In a significant milestone for quantum communication technology, an experiment has demonstrated how networks can be leveraged to combat disruptive ‘noise’ in quantum communications. The international effort led by researchers…

Stretchable quantum dot display
Intrinsically stretchable quantum dot-based light-emitting diodes achieved record-breaking performance. A team of South Korean scientists led by Professor KIM Dae-Hyeong of the Center for Nanoparticle Research within the Institute for…

Internet can achieve quantum speed with light saved as sound
Researchers at the University of Copenhagen’s Niels Bohr Institute have developed a new way to create quantum memory: A small drum can store data sent with light in its sonic…





















