New approach to fight tuberculosis
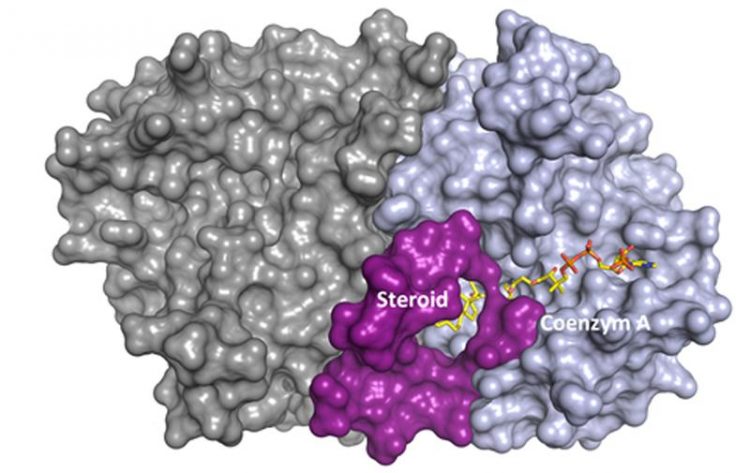
Interaction of the steroid (yellow) with the FadA5 enzyme of the tuberculosis bacillus. The steroid is a good basis for developing a new inhibiting drug. (Image: Caroline Kisker)
In 2012, there were around 8.6 million cases of tuberculosis worldwide resulting in 1.3 million associated deaths according to the World Health Organisation WHO. About five percent of infections were caused by multidrug-resistant pathogens, a trend that is on the rise.
Scientists are therefore seeking new effective ways to tackle the tuberculosis bacteria in the future. Professor Caroline Kisker and her team have devoted their research to this topic: At the University of Würzburg’s Rudolf-Virchow-Center for Experimental Biomedicine, they are studying the bacterial enzymes to pinpoint new vulnerable points.
Enzyme-steroid interaction opens up new prospects
The Würzburg researchers are looking into the pathogens' cholesterol metabolism among others. The enzyme FadA5 is of major interest in this context as it is needed by the bacillus to keep up chronic infection. Teaming up with researchers of Stony Brook University (US), Kisker and her team have now analysed the exact structure of the enzyme – and identified a potential new target for drugs.
“We inserted a steroid molecule into the enzyme's active centre and analysed the resulting structure,” the Würzburg professor explains. This finding helps to design molecules that fit exactly into the active centre and block it with the aim to completely disable the enzyme FadA5, as the research group reports in the January issue of “Structure” journal.
Drug specifically targets the bacterium
A potential problem, however, is that the human organism uses enzymes which are similar to the FadA5 from the tuberculosis bacilli. Hence, it is conceivable that a new drug not only affects the bacteria, but harms the human body as well.
Therefore, Kisker's team analysed the human enzymes, too. The result was promising: “Comparing the structures showed that it should be possible to block the bacterial enzyme specifically,” the professor further. Thus, an inhibiting drug should only harm the bacteria but not the human enzymes.
“The steroid is a solid basis for us to develop new inhibiting drugs,” Kisker says. To pursue this goal, she has teamed up with other work groups, including that of Professor Christoph Sotriffer of the Würzburg Pharmaceutical Chemistry Department. Their aim is to find a drug that specifically inhibits the FadA5 enzyme of the tuberculosis pathogens.
Schaefer et al.: “FadA5 a thiolase from Mycobacterium tuberculosis – a unique steroid-binding pocket reveals the potential for drug development against tuberculosis”, Structure, published online, 2014, December 4, DOI: http://dx.doi.org/10.1016/j.str.2014.10.010
Contact
Prof. Dr. Caroline Kisker, Rudolf Virchow Center for Experimental Biomedicine, University of Würzburg, Phone +49 931 31-80381, caroline.kisker@virchow.uni-wuerzburg.de
Media Contact
More Information:
http://www.uni-wuerzburg.deAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

First-ever combined heart pump and pig kidney transplant
…gives new hope to patient with terminal illness. Surgeons at NYU Langone Health performed the first-ever combined mechanical heart pump and gene-edited pig kidney transplant surgery in a 54-year-old woman…

Biophysics: Testing how well biomarkers work
LMU researchers have developed a method to determine how reliably target proteins can be labeled using super-resolution fluorescence microscopy. Modern microscopy techniques make it possible to examine the inner workings…

Making diamonds at ambient pressure
Scientists develop novel liquid metal alloy system to synthesize diamond under moderate conditions. Did you know that 99% of synthetic diamonds are currently produced using high-pressure and high-temperature (HPHT) methods?[2]…





















