Multiple sclerosis: antibodies can initiate the inflammation at the core of the disease
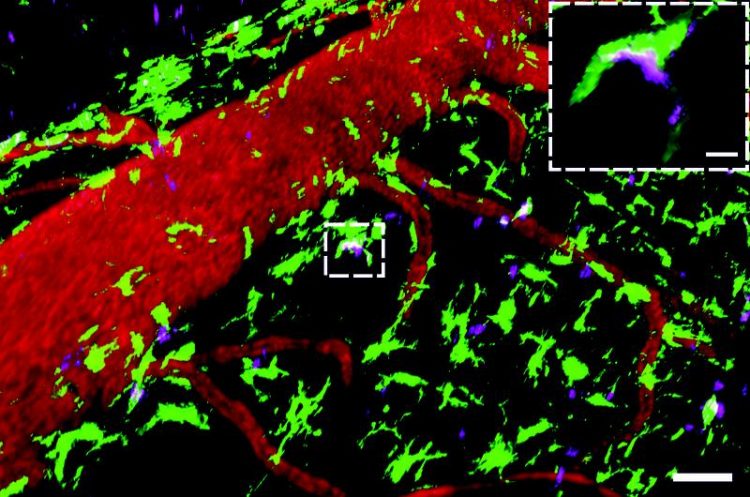
Autoantibodies are concentrated in scavenger cells situated in the meninges Source: umg
Two research groups, one from the Institute of Neuroimmunology / Insti-tute for (UMG), have made a similar discovery independently using an animal model of multiple Multiple Sclerosis Research (IMSF) and the other from the Institute of Neuropathology / Department of Neurology, all belonging to the University Medical Center Göttingen sclerosis: a new mechanism involving so-called autoantibodies that initiates and aggravates inflammatory CNS disease.
This discovery contributes towards understanding what factors bring about multiple sclerosis and, by extension, potential diagnostic and therapeutic op-tions. Publications in the journals “Proceedings of the National Academy of Sciences USA” and “Acta Neuropathologica”.
Original publications: Anne-Christine Flach, Tanja Litke, Judith Strauss, Michael Haberl, César Cordero Gómez, Markus Reindl, Albert Saiz, Hans Jörg Fehling, Jürgen Wienands, Francesca Odoardi, Fred Lühder & Alexander Flügel. Autoanti-body-boosted T cell re-activation in the target organ triggers manifestation of auto-immune CNS disease. Proc. Nat. Acad Sci USA (2016) 13: 3323-3328.
Silke Kinzel, Klaus Lehmann-Horn, Sebastian Torke, Darius Häusler, Anne Winkler, Christine Stadelmann, Natalie Payne, Linda Feldmann, Albert Saiz, Markus Reindl, Patrice H. Lalive, Claude C. Bernard, Wolfgang Brück & Martin S. Weber. Myelin-reactive antibodies initiate T cell-mediated CNS autoimmune disease by opsonization of endogenous antigen. Acta Neuropathol. (2016)
DOI: 10.1007/s00401-016-1559-8
The current understanding of the disease multiple sclerosis is that it is an autoimmune disease, i.e. it is triggered by a sub-group of the body’s own immune cells, so-called T cells. T cells coordinate our immune response and in a healthy person are responsible for defending the body against pathogens and abnormal tumor cells. In the case of multiple sclerosis, a number of T cells mistakenly identify healthy brain tissue as foreign and thereupon start an immune attack against the body’s own central nervous system (CNS).
The collateral damage of this attack is the destruction of nervous tissue, leading to the neurological deficits well-known in multiple sclerosis such as paralysis and somatosensory defects. Why of all things would our T cells attack the brain, a part of the body usually possessing a highly-efficient defense system and sealed off from immune cells? This important question, at the basis of the cause of multiple sclerosis, is as yet largely unanswered. Göttingen researchers have now discovered an immune mechanism that could explain exactly why T cells may focus on brain tissue and then spark off an immune reaction.
Interestingly, our T cells cannot react directly to foreign intruders. Before a T cell activation can take place, with all its potential dramatic consequences, nature has devised an extra control step that must first occur, which is that firstly potential pathogens (e.g. bacteria, tumor cells) must be taken up and digested by scavenger cells. These scavenger cells determine at this stage whether the ingested material could be problematic or is harmless.
The digested “remains”, so-called peptides, are presented to T cells via certain molecules situated on the scavenger cells’ surface. If it is a case of potentially harmful material, then those T cells that recognize the specific peptides in question become activated and raise an alarm. If, however, there is no danger, the T cells do not become activated. Of course the latter should occur if the material in question is the body’s own tissue. Even a healthy body has some T cells that potentially react against the body’s own tissue, but they won’t become activated and cause an immune attack without also receiving the relevant alarm signals.
How is the situation different in multiple sclerosis? The Göttingen researchers discovered that an additional component of the immune system, namely antibodies produced by B cells, play an important role in how brain tissue is taken up by scavenger cells. Antibodies can move freely in bodily fluid, untied to cells. As with T cells, there are some antibodies that target the body’s own tissue, so-called autoantibodies.
These can bind directly to their target natural body tissue. The Göttingen researchers found that in a model of multiple sclerosis where T cells are present that potentially target brain tissue, only the addition of brain tissue-targeting autoantibodies produced a strong activation of the T cells and the resultant fulminant CNS inflammation.
Hereby the autoantibodies bound cell fragments with the relevant target-recognizing structures. As the Göttingen researchers could demonstrate in different model situations, the structures decorated with autoantibodies were eagerly taken up by scavenger cells and, after being digested, presented as self-peptides to T cells. The autoantibodies therefore significantly influenced T cell recognition of the body’s own tissue and their subsequent activation.
Autoantibodies can therefore act as a kind of marker that makes fragments of the body’s own tissue “palatable” to scavenger cells searching in the vicinity. At the same time the autoantibodies also function as “collectors”, because they concentrate in the scavenger cells the number of relatively scarce fragments of the body’s own brain tissue captured by them.
OUTLOOK: WHAT CAN ONE LEARN FROM THE RESULTS OF THIS RE-SEARCH?
The results of this research make clear that autoantibodies can initiate and aggravate the inflammatory process in the nervous system by concentrating the body’s own structures in scavenger cells and thus making them visible to T cells. B cells and autoantibodies have come into focus in multiple sclerosis research, because a therapy targeting B cells has shown remarkable efficacy in treating sufferers of multiple sclerosis. Certainly, though, the exact mechanism by which B cells and autoantibodies play a role in the pathogenesis of multiple sclerosis and therefore the explanation for why the therapy is successful could not be explained.
Up until now it has been assumed that autoantibodies damage tissue structures directly by further destroying already inflamed tissue structures and thereby aggravate a manifest disease. The Göttingen researchers’ data do not rule out this possibility. However, the data points to an additional role for autoantibodies in initiating in multiple sclerosis, a significant piece of knowledge for diagnostic and, above all, therapeutic approaches.
For example, the presence of certain autoantibodies could indicate a risk of an impending acute disease episode and allow specific protective measures to be set in place to avoid it. The key therapeutic potential of the research results surely lie in the fact that the specific group of patients who possess autoantibodies should probably receive a therapy targeting these autoantibodies. This is significant, because the majority of available therapy options in MS target T cells, thus not fighting or not fighting sufficiently strongly the other initiators of the disease.
CAPTION: Autoantibodies are concentrated in scavenger cells situated in the meninges
This real-time microscopic image shows autoantibodies (magenta, autoantibodies specific to the myelin oligodendrocyte glycoprotein (MOG)) binding to scavenger cells (green, CX3CR1 + GFP+ cells) in the meninges. The latter are scattered around blood vessels (red, labelled with the Dextran Texas Red). Scale of overview 50µM, enlarged segment : 20µM. Source: umg
FURTHER INFORMATION:
University Medical Center Göttingen, Georg-August University
Institute of Neuroimmunoloy / Institute for Multiple Sclerosis Research
Alexander Flügel, MD, and Fred Lühder, PhD
Phone +49 (0) 551 / 39-13332, IMSF@uni-goettingen.de
Institute of Neuropathologie / Department of Neurology
Martin S. Weber, MD
Phone +49 () 0551 / 39-12700, neuropat@med.uni-goettingen.de
Media Contact
More Information:
http://www.universitaetsmedizin-goettingen.de/All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

High-energy-density aqueous battery based on halogen multi-electron transfer
Traditional non-aqueous lithium-ion batteries have a high energy density, but their safety is compromised due to the flammable organic electrolytes they utilize. Aqueous batteries use water as the solvent for…

First-ever combined heart pump and pig kidney transplant
…gives new hope to patient with terminal illness. Surgeons at NYU Langone Health performed the first-ever combined mechanical heart pump and gene-edited pig kidney transplant surgery in a 54-year-old woman…

Biophysics: Testing how well biomarkers work
LMU researchers have developed a method to determine how reliably target proteins can be labeled using super-resolution fluorescence microscopy. Modern microscopy techniques make it possible to examine the inner workings…





















