Miniature Pump – Polymer gel continuously responds to fleeting stimuli
<br>
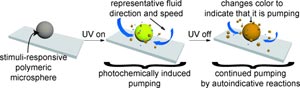
These systems require miniaturized versions of macroscopic components and devices. In the journal Angewandte Chemie, American researchers have now introduced a microscopic pump. It is based on polymer gel microparticles and starts up when irradiated with UV light. The extraordinary thing about this device is that the material continues to pump when the stimulus is removed.
The tiny pumps developed by a team led by Ayusman Sen and Scott T. Phillips at Pennsylvania State University are based on polymer gel spheres with a diameter of 300 µm. Their surface is equipped with two different types of molecules. The first type is split off under UV light, breaking down into CO2, protons, fluoride ions, and a small organic molecule.
The trick is that the fluoride ions cause the second type of molecule to split off of the surfaces of the spheres – even when no UV light is present. The second type of molecule also breaks down into CO2, protons, fluoride ions and a small organic molecule. Because fluoride is constantly being released, the reaction only comes to a halt when all of the type 2 molecules are used up.
How do the spheres “pump”? The molecules and ions they release diffuse away from the surfaces of the spheres and form a concentration gradient. Concentration gradients always produce flow within a liquid: the spheres “suck” the liquid toward themselves. The organic molecule released in the reaction also causes the spheres to change color from white to yellow-orange. This indicates that the micropump is “switched on”.
“Intelligent” polymer materials that can “respond” to an external stimulus with a macroscopic function are the subjects of intensive research. The fact that this material “remembers” the initiating stimulus – the UV light – and continues to pump when it is switched off is something completely new for this type of material. The new material requires no reagents or “fuels” to be added through the liquid. It functions autonomously, converting chemical energy into a mechanical response, the flow of liquid. Molecule 1 receives the signal; the fluoride ions transmit it. This is the first time that all these properties have been combined in an “intelligent” polymeric material.
It should also be possible to devise a similar material that reacts to stimuli other than light, such as the presence of a certain substance. Such microscopic pumps could be used to redirect the flow in a microfluidic system as soon as this specific substance appears.
About the Author
Dr. Scott Phillips is the Martarano Assistant Professor in the Department of Chemistry at Penn State. His areas of interest include developing new strategies for signal amplification, as well as new stimuli-responsive materials and point-of-care diagnostics.
Author: Scott T. Phillips, Pennsylvania State University, University Park (USA), http://www.psu.edu/dept/phillipsgroup/scott.html
Title: A Self-Powered Polymeric Material that Responds Autonomously and Continuously to Fleeting Stimuli
Angewandte Chemie International Edition, Permalink to the article: http://dx.doi.org/10.1002/anie.201304333
Media Contact
More Information:
http://pressroom.angewandte.orgAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…





















