Insights into closed enzymes
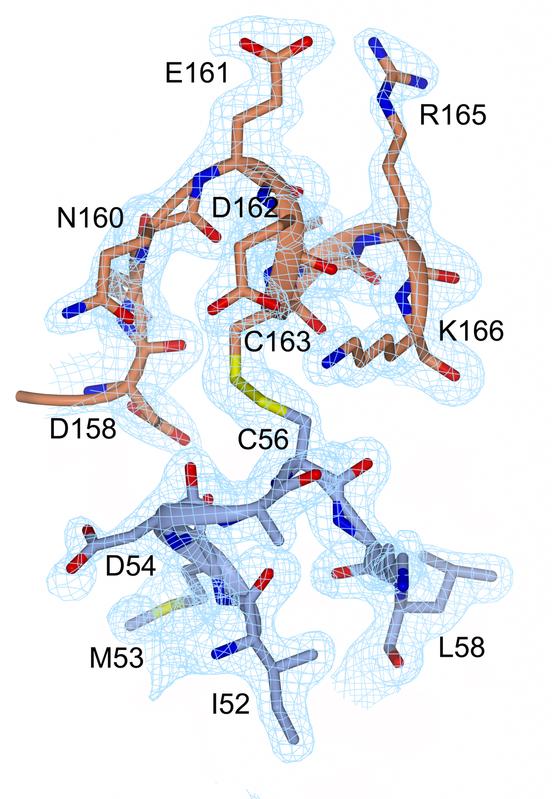
Representation of electron density at the disulphide bond (yellow, between C56 and C163) and in its close vicinity. Prof. Michael Kovermann, University of Konstanz
The adenylate kinase enzyme is crucial to managing the energy budget of cells, accelerating the biochemical process whereby energy is stored or released. The enzyme continuously changes between open and closed states. In its closed form, adenylate kinase is particularly active biochemically and thus able to accelerate the chemical reaction of “docked” molecules that it has encased like a clam.
These are called ligands. Researchers at the Universities of Konstanz (Germany) and Umeå (Sweden) have managed to generate a structural model of the atomic make-up of the enzyme in its closed state, including an embedded ligand. Using nuclear magnetic resonance (NMR) spectroscopy and X-ray crystallography, structural information about the enzyme in its closed state was won – information, that is, about the precise moment that the enzyme is biochemically particularly active.
A particular trick was necessary to facilitate the structural analysis of the enzyme’s closed state in the first place. The research findings, which provide important information about the biochemical mechanisms that govern the energy budgets of cells, were published in the Journal Pro-ceedings of the National Academy of Sciences (PNAS).
The adenylate kinase enzyme opens and closes like a clam: it opens to receive a ligand and closes in order to “process” it biochemically. Afterwards, it opens once again to release it and admit the next ligand. This happens 340 times per second – much too fast to map the individual stages of the process via structural analysis.
For structural biologists, gaining information about the closed state of the enzyme at the precise point in the process where biochemical activity peaks is of immense value. Professor Michael Kovermann, junior professor of magnetic resonance spectroscopy at the University of Konstanz, found a way to make this possible.
He introduced a disulphide bond as a “chemical thread” of sorts in order to force the enzyme to take on its closed form and trap it in this state. The enzyme remains in exactly this position, allowing the researchers to analyse it using NMR spectroscopy and X-ray crystallography. Kovermann was able for the very first time to generate images of the precise moment that the enzyme processes the ligand biochemically.
Two years earlier, he had already managed to take structural images of the enzyme in its open state and with the ligand already in place. “The beauty of it is that, now, we are able to image both liminal states and to make the structural data publicly available”, Kovermann says.
His trick of trapping the enzyme in its closed state can now be used in further inquiries. The state of the enzyme can be controlled by linking or removing the disulphide bond. Kovermann’s analyses had already shown that the enzyme’s affinity for reaction – the chemical pull between the enzyme and its ligand – increases many times over in its closed state, whereas its productive turnover decreases at the same rate. In other words: Chemical activity between ligand and enzyme is particularly high during the closed state. But turnover decreases because the ligand cannot escape the closed “clamshell”, which means fewer ligands in total pass through the enzyme.
Kovermann was also able to prove that adenylate kinase’s structural dynamic strongly depends upon the interaction between enzyme and ligand – i.e. upon the presence or absence of a ligand. To do so, he compared the enzyme’s closed state for both variations, with and without a trapped ligand. If there is no ligand, the closed enzyme’s dynamic remains unchanged as compared to its open state. However, once a ligand is present, marked changes can be observed. “This behaviour is counter-intuitive, it’s not what one would expect”, explains Michael Kovermann. “It is only thanks to NMR spectroscopy that we were able to document this surprising result”.
Original publication:
“Structural basis for ligand binding to an enzyme by a conformational selection pathway”.
M. Kovermann, C. Grundström, E.A. Sauer-Eriksson, U.H. Sauer, M. Wolf-Watz, Proceedings of the National Academy of Sciences 114(24):6298-6303, 2017.
DOI: 10.1073/pnas.1700919114
Facts:
– Direct project funding from: German Research Foundation (DFG), 2013-2015
– Research collaboration with: Umeå University (Sweden)
– The research conducted by Junior Professor Michael Kovermann at the University of Kon-stanz is funded by the Baden-Württemberg Foundation, the Collaborative Research Centre CRC 969 “Chemical and Biological Principles of Cellular Proteostasis” as well as the Kon-stanz Research School Chemical Biology (KoRS-CB).
– Prior publication: “Structural basis for catalytically restrictive dynamics of a high-energy enzyme state”. M. Kovermann, J. Ådén, C. Grundström, E.A. Sauer-Eriksson, U.H. Sauer, M. Wolf-Watz M. Nature Communications, 6:7644, 2015. DOI: 10.1038/ncomms8644.
Note to the editors:
Please click here to download an image:
https://cms.uni-konstanz.de/fileadmin/pi/fileserver/Bilder/Disulfidbr%C3%BCcke.j…
Caption: Representation of electron density at the disulphide bond (yellow, between C56 and C163) and in its close vicinity.
Contact:
University of Konstanz
Communications and Marketing
Phone: + 49 7531 88 -3603
Email: kum@uni-konstanz.de
Media Contact
More Information:
http://www.uni-konstanz.deAll latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Combatting disruptive ‘noise’ in quantum communication
In a significant milestone for quantum communication technology, an experiment has demonstrated how networks can be leveraged to combat disruptive ‘noise’ in quantum communications. The international effort led by researchers…

Stretchable quantum dot display
Intrinsically stretchable quantum dot-based light-emitting diodes achieved record-breaking performance. A team of South Korean scientists led by Professor KIM Dae-Hyeong of the Center for Nanoparticle Research within the Institute for…

Internet can achieve quantum speed with light saved as sound
Researchers at the University of Copenhagen’s Niels Bohr Institute have developed a new way to create quantum memory: A small drum can store data sent with light in its sonic…





















