How cells take out the trash: the “phospho-kiss of death” deciphered
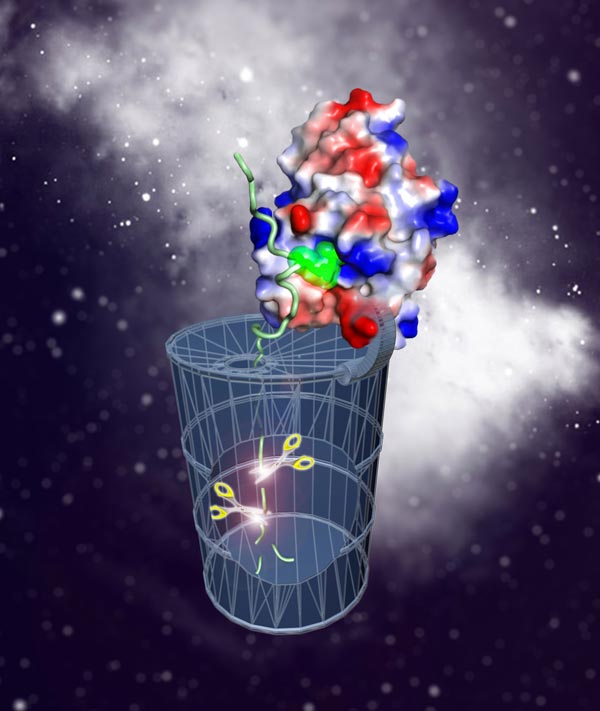
Phosphoarginine functions as a protein degradation tag in Gram-positive bacteria analogous to ubiquitin in eukaryotes
Like in the U2 song title Running to stand still, the apparent steady state of cells belies constant production and removal of proteins within. Although research into protein degradation has lagged behind research into protein synthesis, it is now well appreciated that when it comes to proteins, death is just as important as birth – and as tightly regulated. Each is removed at the right moment and special care is taken to eliminate those that are either damaged or perform functions no longer necessary in a particular context.
The degradation process, which consists of chopping the protein substrate into small fragments, takes place inside compartmentalised proteases – specialised molecular machines often likened to paper shredders. If – as in the eukaryotic ubiquitin-based system – the access to the protease depends on a specific tag that must be carried by the substrate, then the important decision who and when should be eliminated boils down to the timely and selective attachment of tags. The mechanism is elegant, yet scientists working with bacteria have been puzzled to find it used by just one specific branch of their organisms of interest. The current study changes the picture.
Tim Clausen’s lab stumbled upon arginine phosphorylation several years ago while studying how certain bacteria cope with adverse conditions. McsB, a protein involved in this process, turned out to have a unique ability to phosphorylate other proteins on arginine residues. While protein regulation by reversible addition of phosphoryl groups had been the mainstay of biochemistry since the Nobel Prize-winning work of Edwin G. Krebs and Edmond H. Fischer in the 1950s, it was now the first time that it had been observed to involve arginine. Follow-up studies have revealed hundreds of modified proteins in the model organism Bacillus subtilis. Although the modification was observed to be regulated in response to environmental conditions, the function has remained elusive.
“There were several reasons why we thought phosphoarginine might be the ‘bacterial ubiquitin’,” says Tim Clausen, for whom protein degradation has long been an area of intense focus. McsB has been known to be transcriptionally co-regulated with a major protease of the bacterium B. subtilis, ClpCP, and was once proposed to function as its “adaptor” that brings in a specific substrate. Tim speculated that it might in fact work more like a “labeler”, tagging many diverse proteins with a “phospho-kiss of death”. In a series of sophisticated experiments, his group proved the hypothesis to be correct.
“I knew we were on the right track when phosphoarginine showed a strong binding affinity to ClpCP,” says Débora Broch Trentini, a postdoc in the Clausen lab and the first author of the Nature paper. It is this interaction, she adds, that accounts for specificity: proteins marked with phosphoarginine are directly captured by the ClpCP protease and shredded to pieces.
As the discovered system is widely conserved across gram-positive bacteria – a large class that includes the notorious human pathogen Staphylococcus aureus – the study explains previous reports that identified McsB and ClpC as virulence factors. To infect, bacteria must overcome stresses encountered within the host and this in turn relies on efficient degradation of stress-damaged proteins. The discovery from Tim’s lab therefore opens a new perspective on how bacteria-mediated disease could be combated.
“We identified the mechanism. The next step is to block it,” concludes Tim Clausen.
http://www.nature.com/nature/journal/vaap/ncurrent/full/nature20122.html
- Full bibliographic informationTrentini, DB, et al. (2016) “Arginine phosphorylation marks proteins for degradation by a Clp protease”. Nature Accelerated Article Preview, October 6, 2016.
For further information, please contact:
Heidemarie Hurtl
+43 1 79730 358
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…





















