Great strides for carbon capture using earth-abundant elements as photocatalytic system
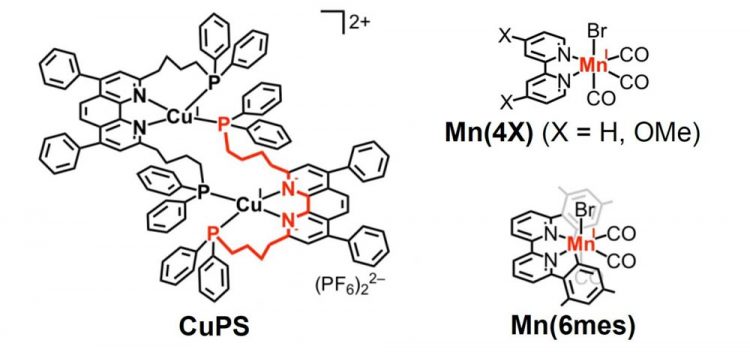
This is the structure of CuPS, the copper complex that behaves as a redox photosensitizer, and the manganese-based catalyst developed in the study. Credit: Journal of the American Chemical Society
As global warming presents one of the biggest challenges to humanity in the 21st century, the quest to curb mounting CO2 emissions is more pressing than ever.
In a study published in the Journal of the American Chemical Society, Osamu Ishitani and colleagues at Tokyo Institute of Technology (Tokyo Tech) and Japan's National Institute of Advanced Industrial Science and Technology report a photocatalytic[1] system that brings scientists closer to achieving artificial photosynthesis — the goal of creating a sustainable system similar to the way that plants convert CO2 to useful energy by using earth abundant metals.
Although metal-complex photocatalytic systems have been reported for CO2 reduction, many of them used noble- and/or rare-metal complexes.
Compared to these approaches that utilize rare metals (such as ruthenium and rhenium), the use of earth abundant metals is “greener” and inexpensive, and has thus attracted much interest.
Their new process is made up of two components (see Figure ): 1) a copper complex (CuPS) that behaves as a redox photosensitizer[2] and 2) a manganese-based catalyst, Mn(4OMe).
CuPS proved to be a stable and efficient redox photosensitizer, as decomposition was only 2% after 12 hours of irradiation. In addition, CuPS exhibited a much stronger reduction capability compared to other photosensitizers investigated to date.
The team reported that the total quantum yield of CO2 reduction products was 57%, the turnover number based on the manganese catalyst was over 1300 and the selectivity of CO2 reduction was 95%.
In particular, the figure of 57% is remarkable, as the researchers comment: “To the best of our knowledge, this is the highest quantum yield for CO2 reduction using abundant elements and the yield would be comparable to that obtained with rare metals.”
The study highlights the way that incremental advances in chemistry may have a large impact on the wider goal of working towards a fossil-fuel-free future.
###
The research was supported by the Japan Science and Technology Agency's CREST program aimed at accelerating strategic innovation.
Technical terms
[1] Photocatalytic: Referring to a light-driven process that can accelerate a particular reaction of interest.
[2] Redox photosensitizer: A component that initiates the photochemical one-electron transfer from a reductant to a catalyst.
Related links
Osamu Ishitani – Seeking photocatalysts for chemical energy https:/
Ishitani-Maeda Laboratory http://www.
Reducing CO2 with common elements and sunlight https:/
[Mn(bipyridyl)(CO)3Br]: An Abundant Metal Carbonyl Complex as Efficient Electrocatalyst for CO2 Reduction https:/
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Superradiant atoms could push the boundaries of how precisely time can be measured
Superradiant atoms can help us measure time more precisely than ever. In a new study, researchers from the University of Copenhagen present a new method for measuring the time interval,…

Ion thermoelectric conversion devices for near room temperature
The electrode sheet of the thermoelectric device consists of ionic hydrogel, which is sandwiched between the electrodes to form, and the Prussian blue on the electrode undergoes a redox reaction…

Zap Energy achieves 37-million-degree temperatures in a compact device
New publication reports record electron temperatures for a small-scale, sheared-flow-stabilized Z-pinch fusion device. In the nine decades since humans first produced fusion reactions, only a few fusion technologies have demonstrated…





















