Gene therapy helps functional recovery after stroke
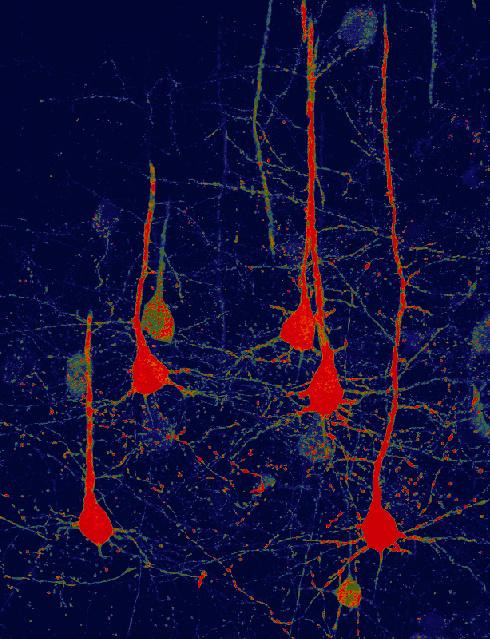
This is an image of neurons (red) that were converted from glial cells using a new NeuroD1-based gene therapy in a stroke-injured mouse brain. Credit: Chen Laboratory, Penn State
“The current treatment for stroke has a narrow time window, typically within a few hours after the occurrence of stroke,” said lead author Yuchen Chen, a postdoctoral fellow at Penn State.
“Many patients cannot receive the treatment in time and as a result, often suffer from permanent disability caused by irreversible neuronal loss. There is an urgent need to develop a new therapy to regenerate new neurons and restore lost brain functions among stroke patients.”
The human brain has approximately 86 billion neurons. While mini-strokes can be tolerated, moderate stroke involving the loss of billions of neurons leaves detrimental effects that do not spontaneously recover.
“So, the critical question that is still unanswered in the neuroregeneration field is how can we regenerate billions of new neurons in a patient's brain after stroke?” said Gong Chen, professor of biology and Verne M. Willaman Chair in Life Sciences at Penn State and leader of the research team.
“The biggest obstacle for brain repair is that neurons cannot regenerate themselves. Many clinical trials for stroke have failed over the past several decades, largely because none of them can regenerate enough new neurons to replenish the lost neurons.”
Gong Chen and his team pioneered a new approach to regenerate functional neurons using glial cells, a group of cells surrounding every single neuron in the brain that provide essential support to neurons. Unlike neurons, glial cells can divide and regenerate themselves, especially after brain injury.
“I believe that turning glial cells that are already present in the brain into new neurons is the best way to replenish the lost neurons,” said Gong Chen. “These glial cells are the neighbors of the dead neurons in the brain and are likely to share the same ancestral cellular lineage.”
Gong Chen's team previously reported that a single genetic neural factor, NeuroD1, could directly convert glial cells into functional neurons inside mouse brains with Alzheimer's disease, but the total number of neurons generated was limited.
The research team believed that this limited regeneration was due to the retroviral system used to deliver NeuroD1 to the brain. In the current study, the research team used the AAV viral system, which is now the first choice for gene therapy in the nervous system, to deliver NeuroD1 into mouse motor cortex that had suffered from stroke.
Many neurons die after stroke but surviving glial cells can proliferate and form a glial scar in the stroke areas. The AAV system was designed to express NeuroD1 preferentially in the glial cells that form these scars, turning them directly into neuronal cells. Such direct glia-to-neuron conversion technology not only increased neuronal density in the stroke areas, but also significantly reduced brain tissue loss caused by the stroke.
Interestingly, the newly converted neurons showed similar neuronal properties to the neurons that were lost after stroke. This suggests a potential impact of the local glial lineage on the converted neuronal identity.
“The most exciting finding of this study is to see the newly converted neurons being fully functional in firing repetitive action potentials and forming synaptic networks with other preexisting neurons,” said Gong Chen. “They also send out long-range axonal projections to the right targets and facilitate motor functional recovery.”
A separate collaborative work led by Gregory Quirk, professor at the University of Puerto Rico, further tested the NeuroD1-based gene therapy in a rat stroke model. Quirk and colleagues also found that this direct glia-to-neuron conversion technology can rescue cognitive functional deficits induced by stroke.
“Because glial cells are everywhere in the brain and can divide to regenerate themselves, our study provides the proof-of-concept that glial cells in the brain can be tapped as a fountain of youth to regenerate functional new neurons for brain repair not only for stroke but also for many other neurological disorders that result in neuronal loss,” said Yuchen Chen. “Our next step is to further test this technology and ultimately to translate it into clinically effective therapies to benefit millions of patients worldwide.”
###
In addition to Chen, Chen, and Quirk, the research team includes Ningxin Ma, Zifei Pei, Zheng Wu, Susan Keefe, Emma Yellin, Miranda Chen, Jiuchao Yin, Grace Lee, Yi Hu, Yuting Bai, and Kathryn Lee at Penn State and Fabricio H. Do-Monte and Angélica Minier-Toribio at the University of Puerto Rico. The research was supported by the U.S. National Institutes of Health and the Penn State Charles H. Skip Smith Endowment Fund to Gong Chen.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

“Nanostitches” enable lighter and tougher composite materials
In research that may lead to next-generation airplanes and spacecraft, MIT engineers used carbon nanotubes to prevent cracking in multilayered composites. To save on fuel and reduce aircraft emissions, engineers…

Trash to treasure
Researchers turn metal waste into catalyst for hydrogen. Scientists have found a way to transform metal waste into a highly efficient catalyst to make hydrogen from water, a discovery that…

Real-time detection of infectious disease viruses
… by searching for molecular fingerprinting. A research team consisting of Professor Kyoung-Duck Park and Taeyoung Moon and Huitae Joo, PhD candidates, from the Department of Physics at Pohang University…





















