Gatekeeping proteins to aberrant RNA: You shall not pass
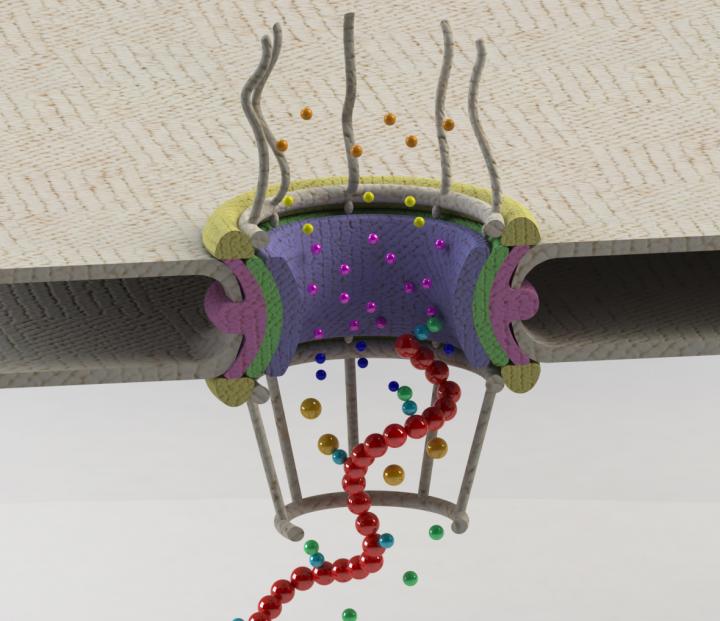
Schematic of a gateway in the nuclear membrane, known as the nuclear pore complex (NPC), and the proteins (shown as spheres) involved in transport and quality control of mRNAs (shown in red). A combination of a multitude of protein-protein interactions enables the cell to distinguish and keep aberrant Credit: Mohammad Soheilypour/Berkeley Lab
Mistakes happen. This is the case in the process of transporting genetic information in cells. How our cells keep errors in this process in check is the subject of a new paper by researchers at the Department of Energy's Lawrence Berkeley National Laboratory (Berkeley Lab).
They found that proteins associated with aberrant strands of genetic code are regulated such that they enable gateway proteins to recognize and block them from exiting the nucleus. Unused messenger RNA (mRNA) strands that cannot exit the nucleus would eventually disintegrate.
Their findings, to be published Wednesday, Nov. 2, in the journal Scientific Reports, shed light on a complex system of cell regulation that acts as a form of quality control for the transport of genetic information out of the nucleus.
Getting a more complete picture of how genetic information gets expressed in cells is important in disease research, the researchers said.
“Some components of this machinery are dysregulated in various types of cancers,” said study principal investigator Mohammad Mofrad, faculty scientist at Berkeley Lab's Molecular Biophysics and Integrated Bioimaging Division. “Understanding the molecular mechanism of genetic information transport and quality control would substantially improve the current knowledge about various types of cancers and other human diseases.”
Biology textbooks already describe how strands of mRNA copy sections of DNA inside a cell's nucleus and then exit to the cell's cytoplasm. It is in the cytoplasm where the genetic code is used to synthesize proteins, so ensuring that only the correct mRNA strands get used is critical to the formation of properly functioning proteins.
“Just like all production lines, the process of genetic information transfer and protein production is quality controlled at different stages,” said Mofrad. “To date, the exact mechanism of this quality control step has remained unclear.”
Previous studies have looked at specific steps in this process, but the complex system of sorting out RNA that is ready to leave the nucleus has not been well understood.
Mofrad, who is also a professor of bioengineering and of mechanical engineering at UC Berkeley, and his Ph.D. student, Mohammad Soheilypour, turned to a computer model to shed light on this process of mRNA export.
“With experiments, we can study parts of a system, but there are limitations to their ability to provide the level of spatial and temporal resolution we need to really understand the behavior of a whole system,” said Soheilypour.
The researchers focused on the interactions of messenger RNA, RNA-binding proteins, and gateway proteins called “nuclear basket proteins.”
For humans and other vertebrates, these nuclear basket proteins are called Tpr, and for yeast they are Mlp1 and Mlp2. The nuclear basket proteins are positioned like guards at the membrane's gateways– the nuclear pore complex (NPC)–through which mRNA must pass to leave the nucleus.
After validating the computer model with known data from previous studies, the researchers ran simulations to test the factors that influence the transport of mRNA out of the nucleus.
They found that a combination of a multitude of protein-protein interactions enables the cell to verify the readiness of mRNA for transport out of the nucleus. RNA-binding proteins are attached to each strand of mRNA, helping to recruit export receptors. Researchers found that regulation of the interaction between RNA-binding proteins and export receptors is the key for nuclear basket proteins to distinguish aberrant mRNAs and retain them inside the nucleus.
“Imagine that in order to exit the gate, you need a certain number of validated tickets,” said Soheilypour. “The RNA-binding proteins are like the tickets the mRNA needs to get out, but those tickets need to be validated by the export factors. Without enough validated tickets, the guard proteins do not recognize the mRNA strand as something to let pass through the membrane's gate.”
The study also found that longer strands of mRNA have more trouble passing through the nuclear membrane. They theorize that because longer mRNA needs extra time to compact itself while trying to get through the gate, guard proteins have more chances to check for “validated tickets.”
More factors in this system may be considered in future studies, the researchers said.
###
The National Science Foundation helped support this research. Simulations were conducted on a computer cluster partly funded by Intel Corp.
Lawrence Berkeley National Laboratory addresses the world's most urgent scientific challenges by advancing sustainable energy, protecting human health, creating new materials, and revealing the origin and fate of the universe. Founded in 1931, Berkeley Lab's scientific expertise has been recognized with 13 Nobel prizes. The University of California manages Berkeley Lab for the U.S. Department of Energy's Office of Science. For more, visit http://www.
DOE's Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















