Formation of the browning pigment melanin decoded
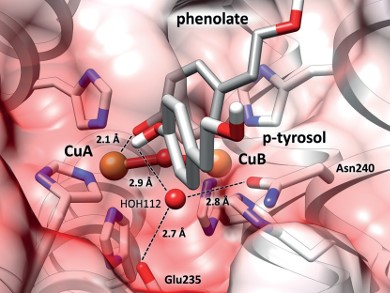
View into the catalytic center of a tyrosinase: The two amino acids Glu235 and Asn240 bind to a water molecule HOH112, which strips a proton (white) away from the substrate (p-tyrosol). The resulting phenolate can now bind to the copper ion (CuA), starting the tyrosinase reaction. Image/©: Institute of Molecular Biophysics
Melanin is a pigment which is present in almost all life forms and that determines hair and skin color in humans. It helps insects protect themselves against the effects of pathogenic microorganisms and it promotes tissue repair. The dark spots on fruits such as bananas can be attributed to the presence of melanin.
However, the processes involved in the formation of this pigment were not yet fully understood. Researchers at the universities in Mainz and Kiel have now uncovered the molecular mechanism underlying melanin synthesis using a clever biotechnological procedure. With this, a major gap in our understanding of how this enzyme functions has been closed.
At the core of the mechanism is the activity of the enzyme tyrosinase. This discovery opens the door to the development of numerous applications in the cosmetics and food industries as well as in environmental technology and medicine.
Tyrosinase initiates the melanin synthesis process. “We previously did not fully understand the role played by this enzyme. In fact, we knew more about the activities of catechol oxidase, a related but less potent enzyme that is also involved in the synthesis of melanin,” explained Heinz Decker, Director of the Institute of Molecular Biophysics at Johannes Gutenberg University Mainz (JGU).
Much research on the cause for the difference in the reactivity of tyrosinase and catechol oxidase has been conducted over the past few decades, but little success had been achieved to date.
Following up on clues from reported research undertaken by an Israeli team led by Dr. A. Fishman, Professor Heinz Decker and Even Solem of Mainz University and Professor Felix Tuczek of Kiel University decided to conduct experiments to discover the mechanism responsible for tyrosinase activity. They first isolated a catechol oxidase from Riesling wine leaves and converted it to a tyrosinase by means of a biotechnological process involving targeted mutation.
They found that the difference in reactivity is attributable to two amino acids, a highly conserved glutamic acid and asparagine that are located near the catalytic center. They form such a strong bond with a specific water molecule within the protein matrix that the water molecule undergoes a charge displacement.
This makes one side strongly negative, so that it strips a positive proton from a nearby monophenol. This then activates tyrosinase which converts the monophenols to chemically very reactive substances called quinones, which combine on their own to form melanin. However, in the absence of asparagine or a water molecule in the protein, only catechol oxidase is present and no tyrosinase.
This discovery is a major breakthrough in the understanding of the catalytic role played by tyrosinase in the synthesis of melanin. This means that in the future it will be possible to make systematic improvements in the processes of stimulation, inhibition, and modification as well as in biotechnological methods employed in medicine, cosmetics production, and in environmental research, with the help of genetically-based approaches.
“In addition, we have gained further insights into the functioning of copper in the body,” concluded Decker. The results of the study have been published in the journal Angewandte Chemie International Edition.
Publication:
Even Solem, Felix Tuczek, Heinz Decker
Tyrosinase versus Catechol Oxidase: One Asparagine Makes the Difference
Angewandte Chemie International Edition, 15 January 2016
DOI: 10.1002/anie.201508534
Image:
http://www.uni-mainz.de/bilder_presse/10_biophysik_tyrosinase.jpg
View into the catalytic center of a tyrosinase: The two amino acids Glu235 and Asn240 bind to a water molecule HOH112, which strips a proton (white) away from the substrate (p-tyrosol). The resulting phenolate can now bind to the copper ion (CuA), starting the tyrosinase reaction.
Image/©: Institute of Molecular Biophysics
Further information:
Professor Dr. Heinz Decker
Institute of Molecular Biophysics
Johannes Gutenberg University Mainz
55099 Mainz, GERMANY
phone +49 6131 39-23570
fax +49 6131 39-23557
e-mail: hdecker@uni-mainz.de
http://www.biophysik.uni-mainz.de/
http://onlinelibrary.wiley.com/wol1/doi/10.1002/anie.201508534/full
Media Contact
All latest news from the category: Life Sciences and Chemistry
Articles and reports from the Life Sciences and chemistry area deal with applied and basic research into modern biology, chemistry and human medicine.
Valuable information can be found on a range of life sciences fields including bacteriology, biochemistry, bionics, bioinformatics, biophysics, biotechnology, genetics, geobotany, human biology, marine biology, microbiology, molecular biology, cellular biology, zoology, bioinorganic chemistry, microchemistry and environmental chemistry.
Newest articles

Properties of new materials for microchips
… can now be measured well. Reseachers of Delft University of Technology demonstrated measuring performance properties of ultrathin silicon membranes. Making ever smaller and more powerful chips requires new ultrathin…

Floating solar’s potential
… to support sustainable development by addressing climate, water, and energy goals holistically. A new study published this week in Nature Energy raises the potential for floating solar photovoltaics (FPV)…

Skyrmions move at record speeds
… a step towards the computing of the future. An international research team led by scientists from the CNRS1 has discovered that the magnetic nanobubbles2 known as skyrmions can be…





















